A pharmaceutical composition containing fasudil hydrochloride for injection
A technology of fasudil hydrochloride and its composition, which is applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of poor stability, quality reduction, and difficulty in light inspection of injections, and achieve Solve the risk of human blood vessel stimulation, inhibit the generation of degraded impurities, and have high industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
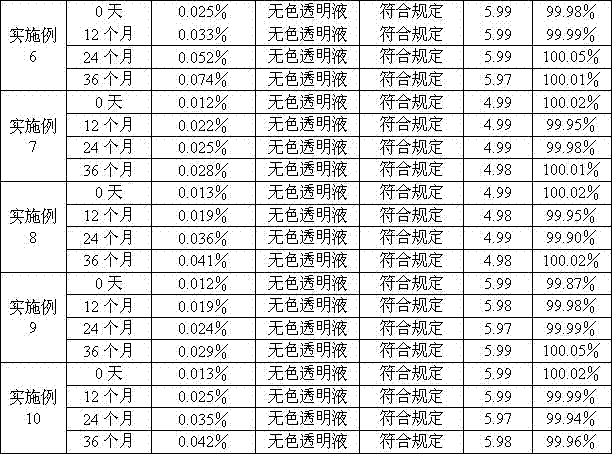
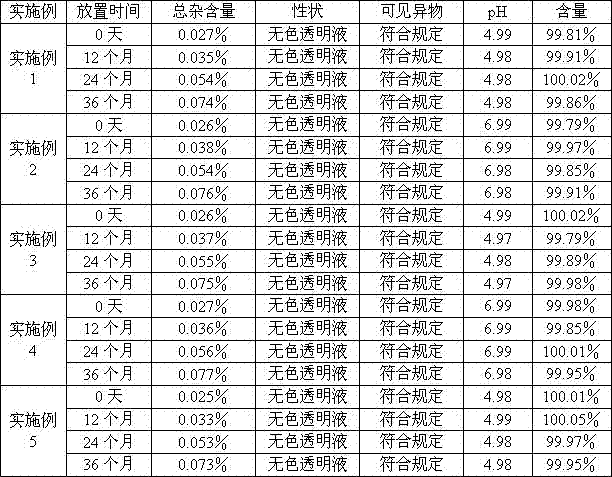
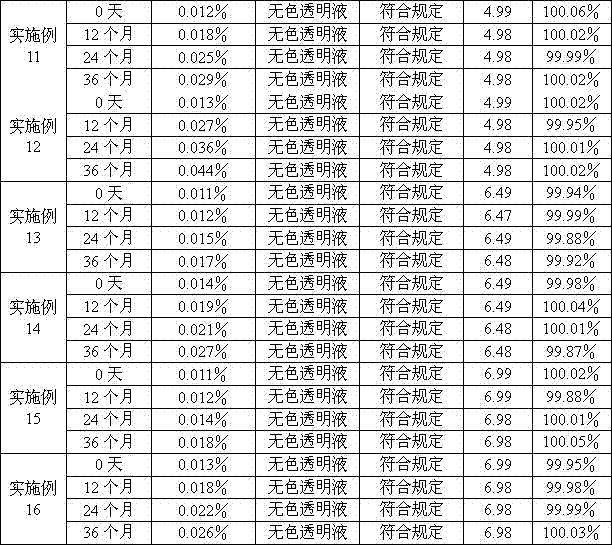
Examples
Embodiment 1
[0131] prescription:
[0132] Fasudil Hydrochloride 25g
[0133] Citric acid 0.5g
[0134] Sodium Citrate 2.0g
[0135] Water for injection was added to 2000ml.
[0136] Preparation method: add 80% of the prescription water for injection into the liquid preparation tank, then add the prescription of fasudil hydrochloride, citric acid and sodium citrate, after stirring and dissolving, add 0.05% of activated carbon for injection, stir evenly, Adsorb at room temperature for 10 minutes, filter to remove carbon, add water for injection to the full amount, stir evenly, adjust the pH value to 5.0 with 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution, and filter through 0.45μm after the semi-finished product is qualified. , filtered through a 0.22μm filter element, filled in a brown low borosilicate glass ampoule bottle, sterilized in a water bath at 121°C for 15 minutes, and inspected by light. 500 samples were made.
Embodiment 2
[0138] prescription:
[0139] Fasudil Hydrochloride 35g
[0140] Citric acid 2.0g
[0141] Sodium Citrate 9.0g
[0142] Water for injection was added to 2000ml.
[0143] Preparation method: add 80% of the prescription water for injection into the liquid preparation tank, then add the prescription of fasudil hydrochloride, citric acid and sodium citrate, after stirring and dissolving, add 0.05% of activated carbon for injection, stir evenly, Adsorb at room temperature for 10 minutes, filter to remove carbon, add water for injection to the full amount, stir evenly, adjust the pH value to 7.0 with 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution, and filter through 0.45μm after the semi-finished product is qualified. , finely filtered through a 0.22μm filter element, filled in a brown medium borosilicate glass ampoule bottle, sterilized in a water bath at 121°C for 15 minutes, and inspected by light. 1000 samples were made.
Embodiment 3
[0145] prescription:
[0146] Fasudil Hydrochloride 25g
[0147] Citric acid 2.0g
[0148] Sodium Citrate 2.0g
[0149] Water for injection was added to 2000ml.
[0150] Preparation method: add 80% of the prescription water for injection into the liquid preparation tank, then add the prescription of fasudil hydrochloride, citric acid and sodium citrate, after stirring and dissolving, add 0.05% of activated carbon for injection, stir evenly, Adsorb at room temperature for 10 minutes, filter to remove carbon, add water for injection to the full amount, stir evenly, adjust the pH value to 5.0 with 0.1mol / L hydrochloric acid solution or 0.1mol / L sodium hydroxide solution, and filter through 0.45μm after the semi-finished product is qualified. , filtered through a 0.22μm filter element, filled in a brown low borosilicate glass ampoule bottle, sterilized in a water bath at 121°C for 15 minutes, and inspected by light. 1000 samples were made.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com