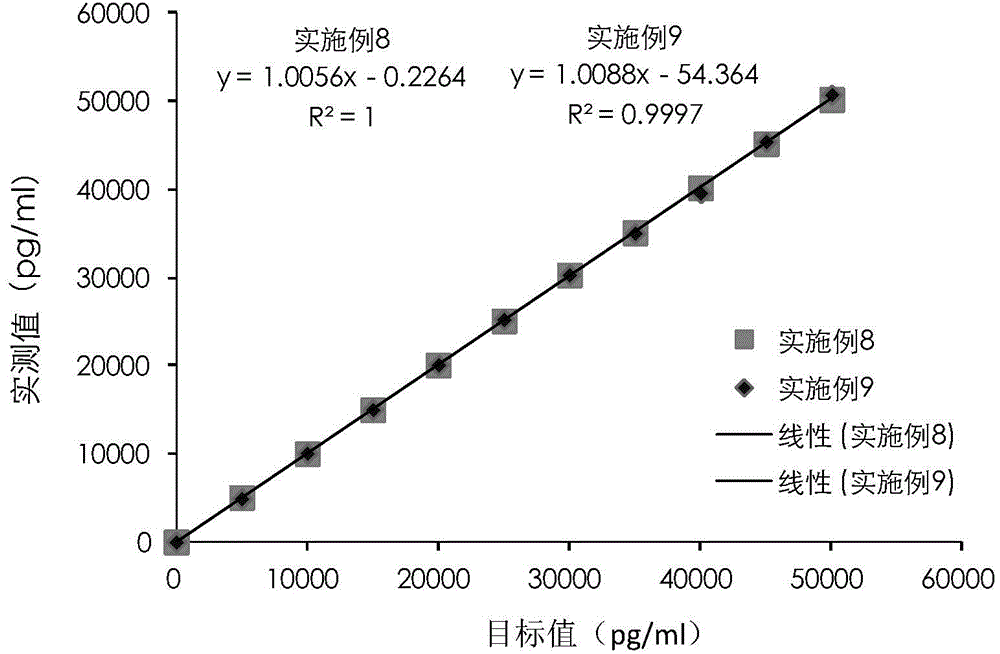
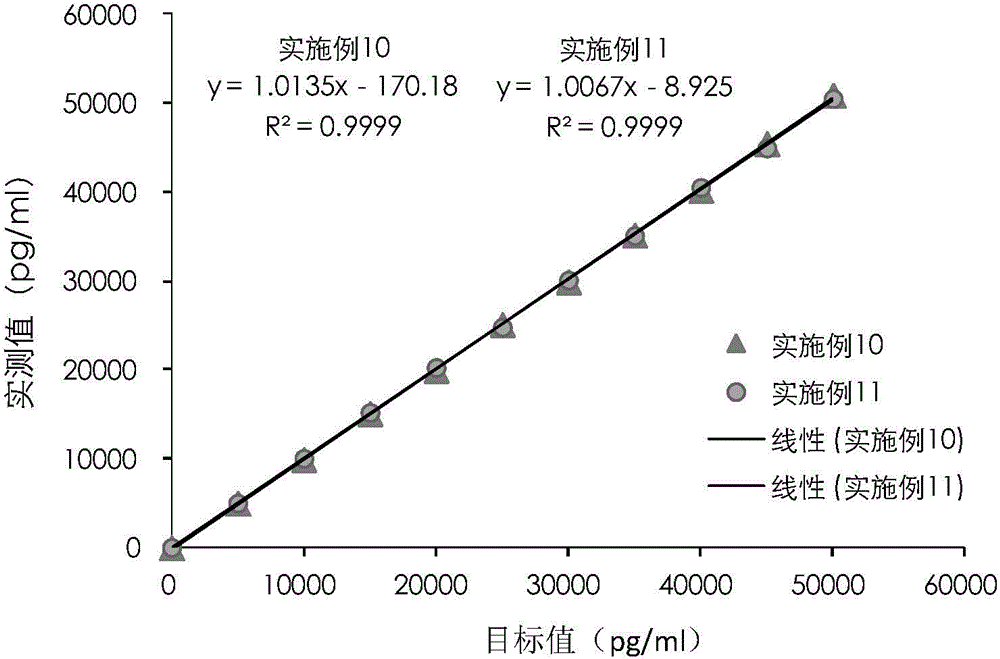
Cardiac troponin I (cTn I) hypersensitive detection kit and hypersensitive detection method
A technique for cardiac troponin and detection kit, which is applied in biological tests, material inspection products, etc., can solve problems such as insufficient sensitivity for clinical application, inability to accurately detect cTnI, and inability to meet early diagnosis of AMI.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Kit 1 was prepared by the following steps:
[0127] Two strains of cTnI antibodies with amino acid segments 18-28 and 83-93 capable of binding to cTnI were used as the first anti-cTnI antibody, and were labeled with ABEI according to the above-mentioned 4 steps of preparation to obtain the first anti-cTnI antibody solution labeled with ABEI. Wherein, the total concentration of the first anti-cTnI antibody is 300ng / mL, and the concentration of ABEI is 30pg / mL.
[0128] Two strains of cTnI antibodies with cTnI-binding amino acid segments 41-49 and 190-196 were used as the second anti-cTnI antibody, and magnetic microspheres were coated according to the above-mentioned steps of Preparation 1 to obtain the second antibody coated with magnetic microspheres. cTnI antibody solution. Wherein, the total concentration of the second anti-cTnI antibody is 5 μg / mL, and the concentration of the magnetic microspheres is 1 mg / mL.
[0129] Dilutions were prepared as in Preparation 11....
Embodiment 2
[0133] Kit 2 was prepared by the following steps:
[0134] Two strains of cTnI antibodies with amino acid segments 26-35 and 83-93 capable of binding to cTnI were used as the first anti-cTnI antibody, and labeled with ABEI according to the above-mentioned 4 steps of preparation to obtain the first anti-cTnI antibody solution labeled with ABEI. Among them, the total concentration of the first anti-cTnI antibody was 300 ng / mL, and the ABEI concentration was 30 pg / mL.
[0135] Two strains of cTnI antibodies with cTnI-binding amino acid segments 41-49 and 169-178 were used as the second anti-cTnI antibody, and a biotinylated second anti-cTnI antibody solution was prepared according to the above-mentioned steps in Preparation 5. Among them, the total concentration of the second anti-cTnI antibody was 300 ng / mL, and the biotin concentration was 30 pg / mL.
[0136] The streptavidin solution coated with magnetic microspheres was prepared according to Preparation 2 above. Wherein, the...
Embodiment 3
[0141] The magnetic microsphere suspension coated with goat anti-FITC polyclonal antibody was prepared according to the above-mentioned preparation 3.
[0142] Using the first anti-cTnI antibody in Example 1, the first anti-cTnI antibody solution labeled with ABEI was prepared according to the above-mentioned Preparation 4.
[0143] Using the second anti-cTnI antibody in Example 1, a FITC solution labeled with the second anti-cTnI antibody was prepared according to Preparation 6 above.
[0144] Dilutions were prepared as in Preparation 11 above.
[0145] The high and low point calibrator solutions were prepared according to Preparation 12 above.
[0146] Each of the solutions prepared above constitutes the components of the kit 3 provided in this example.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com