Therapeutic agent for systemic bone disease and use thereof
A technology of bone system diseases and pharmacy, applied in bone diseases, applications, drug combinations, etc., can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
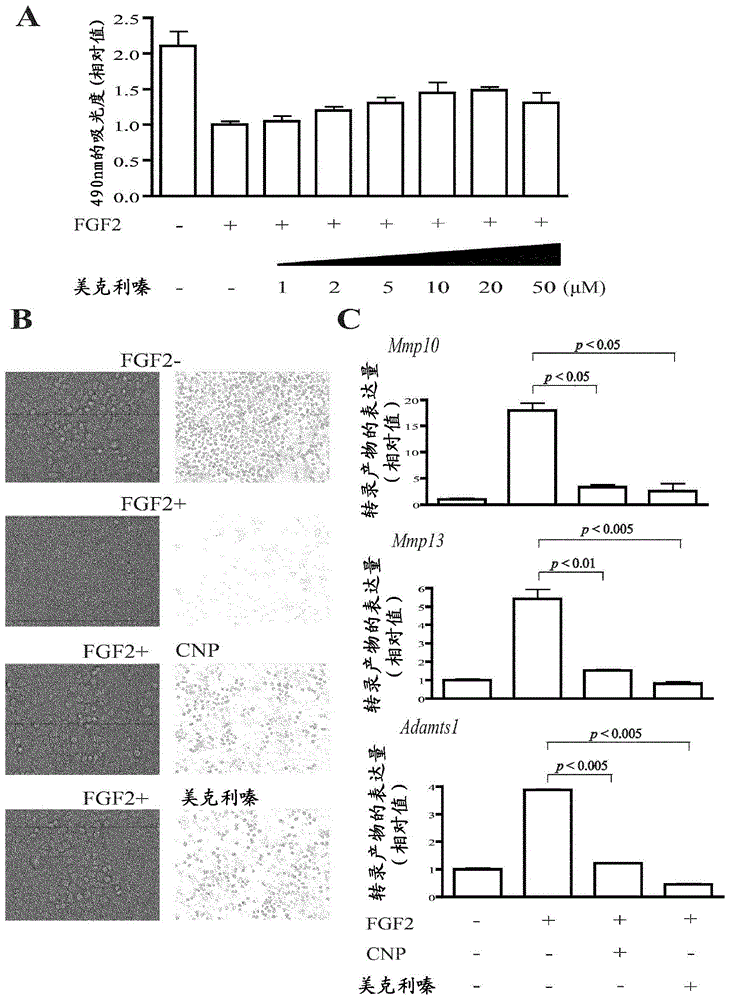
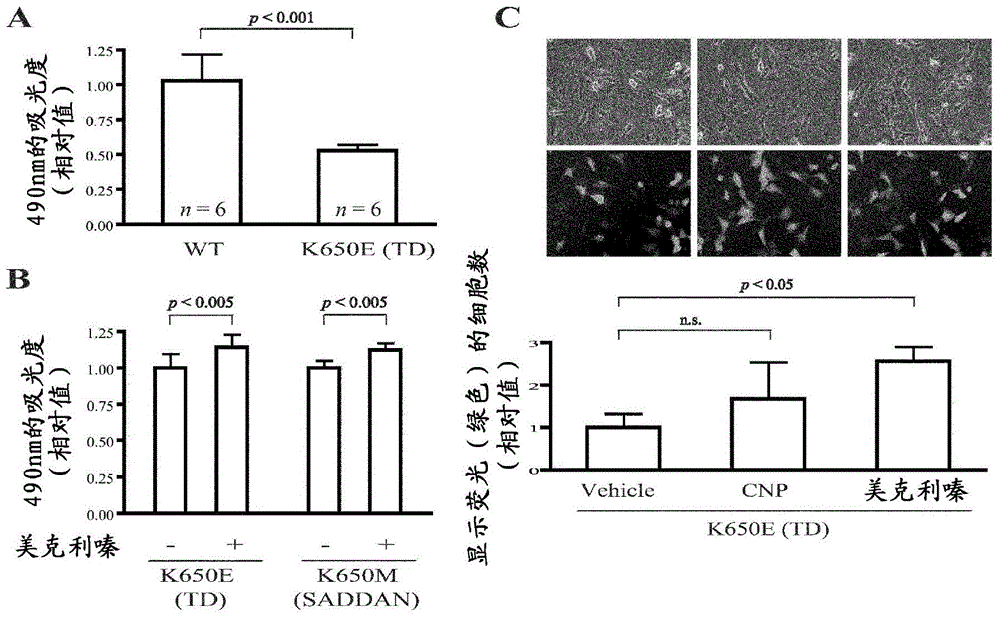
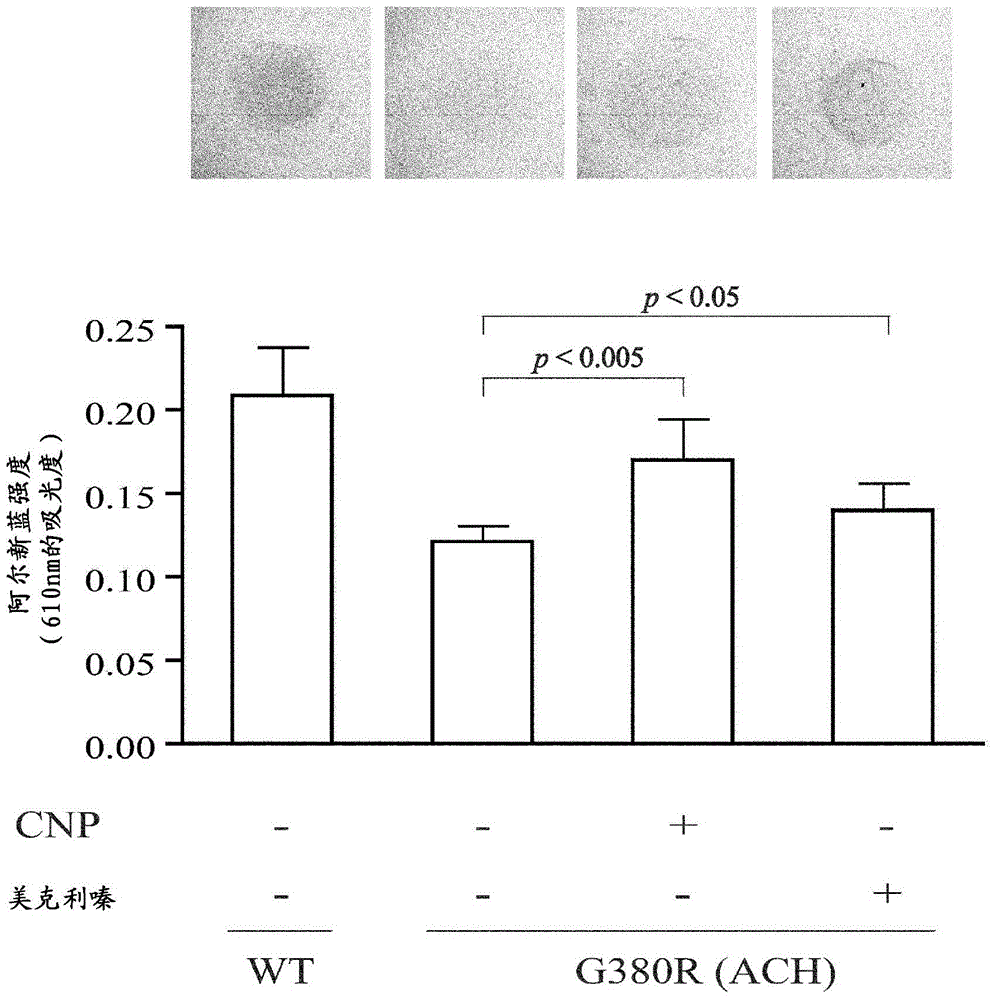
[0051] In recent years, the strategy of exploring new functions of existing drugs and expanding their applications, that is, drug repositioning (drug repositioning) has attracted attention (References 20 and 21). One of the advantages of this strategy is that the optimal dosage, side effects, etc. of the identified drug have been clarified, so it can be immediately applied clinically. In the following, 1186 FDA-approved drugs were screened for the purpose of identifying agents effective in the treatment of achondroplasia (hypoplasia) and other skeletal dysplasia involving FGFR3.
[0052] 1. Materials and Methods
[0053] (1) Screening of 1186 FDA-approved drugs using rat chondrosarcoma (RCS) cells
[0054] RCS cells provided by Dr. Pavel Krejci (Medical Genetics Institute, Cedars-Sinai Medical Center, LA) were cultured in Dulbecco's modified Eagle medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Thermo Scientific) ( Reference 14). For the RSC prolife...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com