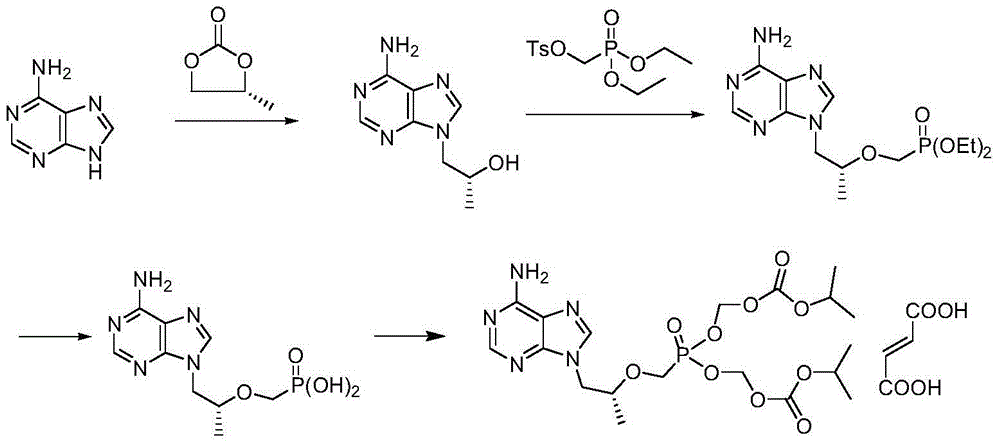
Method for preparing tenofovir disoproxil fumarate
A technology of tenofovir fumarate and disoproxil, applied in the field of medicine, can solve the problem of reducing the total yield of tenofovir fumarate, poor reaction effect of 2-propanediol and affecting product yield. problems such as yield and purity, to achieve the effects of high yield, less side reactions, and improved yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of (R)-4-methyl-1,3-dioxolane-2-one
[0039]Add 33.0g (R)-1,2-propanediol, 0.8g sodium hydroxide, 51.3g diethyl carbonate, and 10ml absolute ethanol into a 500ml three-necked flask, stir, heat up to 95°C for reaction, and detect the reaction by thin-layer chromatography Whether it is complete (the developer is ethyl acetate (V / ml):petroleum ether (V / ml)=3:2; iodine fumigation for color development), after the reaction is complete, heat up to 120°C and continue to evaporate the solvent under reduced pressure to obtain 40.6 g of (R)-4-methyl-1,3-dioxolane-2-one as pale yellow oil, with a purity of 98.0% and a molar yield of 91.6%.
[0040] 1 HNMR (500MHz, CDCl 3 ): δ4.87-4.86(m, 1H), 4.58-4.55(m, J=8.5Hz, 1H), 4.05-4.02(m, J=8.5Hz, 1H), 1.50-1.49(d, J=6.5 Hz, 3H).
Embodiment 2
[0042] (2) Synthesis of (R)-9-(2-hydroxypropyl)adenine
[0043] Add 575ml of N,N-dimethylformamide, 49.5g (R)-4-methyl-1,3-dioxolan-2-one, 45.0g adenine and 0.85g hydrogen to a 2000ml three-necked flask Potassium oxide, stirred, heated to 145°C, kept warm for 22 hours, detected by thin-layer chromatography (developing solvent is dichloromethane (V / ml):methanol (V / ml)=9:1), after confirming that the reaction is complete, Lower the temperature to 90°C and begin to distill off 4 / 5 of the reaction solvent under reduced pressure. After the evaporation is complete, add 672ml of isopropanol, heat up to 80°C and stir to dissolve, then cool down to 0°C and stir for crystallization for 1 hour, then filter and dry the filter cake in vacuum After 8 hours, 57.7 g of white solid was obtained, namely (R)-9-(2-hydroxypropyl)adenine, the molar yield was 85.5%, and the HPLC purity was 98.3%.
[0044] 1 H NMR (500MHz, CDCl 3 ): δ4.87-4.86(m, 1H), 4.58-4.55(m, J=8.5Hz, 1H), 4.05-4.02(m, J=8.5H...
Embodiment 3
[0046] (2) Synthesis of (R)-9-(2-hydroxypropyl)adenine
[0047] Add 575ml of 1 N,N-dimethylformamide, 31.5g (R)-4-methyl-1,3-dioxolan-2-one, 45.0g adenine and 0.85g Potassium hydroxide, stirred, heated to 135°C, kept warm for 22 hours, detected by thin layer chromatography (developing solvent is dichloromethane (V / ml):methanol (V / ml)=9:1), after confirming that the reaction is complete , the temperature was lowered to 80°C, and 4 / 5 of the reaction solvent was evaporated under reduced pressure. After the evaporation was completed, 672ml of isopropanol was added, heated to 70°C and stirred to dissolve, then cooled to 5°C, stirred and crystallized for 1 hour, and suction filtered. The cake was vacuum-dried to obtain 57.7 g of white solid, namely (R)-9-(2-hydroxypropyl)adenine, with a molar yield of 84.7% and an HPLC purity of 97.9%.
[0048] 1 H NMR (500MHz, CDCl 3 ): δ4.87-4.86(m, 1H), 4.58-4.55(m, J=8.5Hz, 1H), 4.05-4.02(m, J=8.5Hz, 1H), 1.50-1.49(d, J=6.5 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com