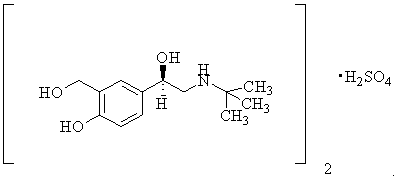
Drug application of L(r) salbutamol preparation in the treatment of skin and mucous membrane wound ulcer
A technology of levalbuterol and skin wound surface, applied in the field of preparations of levalbuterol medicinal salts and combinations thereof, and can solve problems such as unreported levalbuterol and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of Levalbuterol Cream
[0017] The preparation method of levalbuterol cream is as follows: prepare hydrophobic phase: accurately weigh 5% petrolatum, 10% paraffin oil, 5% gelatin, 6% glyceryl monostearate, 0.5 Tween80, heat to 70°C .
[0018] Preparation of the hydrophilic phase: accurately weigh 0.5% levosalbutamol sulfate, 5% propylene glycol, and 0.5% benzyl alcohol, and add water to 73.5%. Heat to 70°C. The two phases are mixed, and the mixture is cooled under stirring, and the levosalbutamol ointment is obtained. The ratio of levosalbutamol and auxiliary materials can also be adjusted according to demand, such as from 0.01%-99%. Salbutamol.
Embodiment 2
[0020] Experimental Study of Levo-Salbutamol Sulfate Ointment on Skin Wounds of Mice
[0021] 60 KM mice weighing 25-29g were randomly assigned to four experimental groups A, B, C, and D, with 15 mice in each group, and an acute trauma model was created in the depilated area of the skin on the back of the mice, using a circular punch A full-thickness skin excision is made, creating a circular hole with a diameter of 8 mm (or two incisions, one on the back and one on the buttocks). Raised in separate cages. Groups A, B, C and D were smeared with self-made levosalbutamol ointment, blank excipients and positive control drug (ofloxacin) respectively. Administration method: Administer once a day (extension to 2-3mm, thickness about 0.5mm).
[0022] Observe the morphological changes of the wound every day, take pictures and measure the diameter of the wound, and record the time of scab formation and healing of the wound. The healing was defined as the wound surface was closed, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com