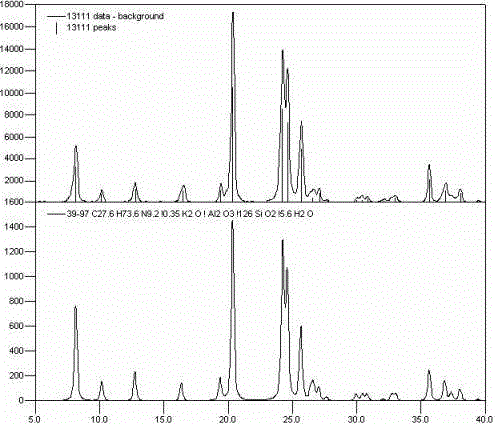
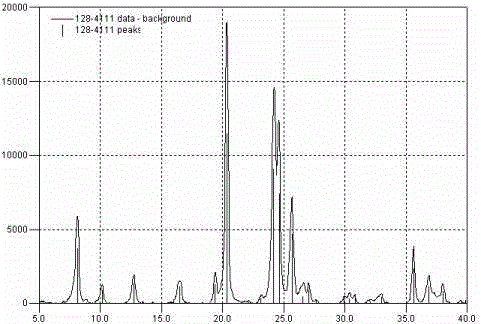
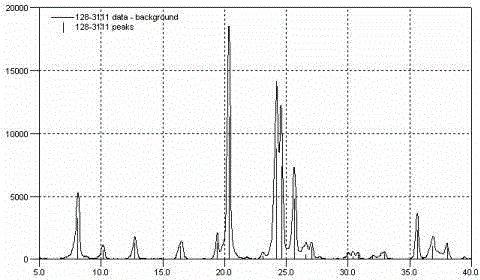
Regeneration method for catalyst containing precious metal and molecular sieve
A catalyst and noble metal technology, applied in the field of deactivated catalyst regeneration, can solve the problems of difficult separation of macromolecular by-products, molecular sieve structure damage, metal platinum aggregation, etc., to improve hydrogenation/dehydrogenation reaction activity, improve dispersion, good Dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Catalyst E-1 of the Invention.
[0033] Add 1450ml of organic solvent (the volume ratio of ethanol and benzene is 2:1) into a 2000ml flask, and carry out deactivation of the catalyst (FD-1, Pt content 0.45 wt%, carbon content 7.24wt% the same below) after industrial operation. After processing, the catalyst number after 2 hours of distillation was Ca-1.
[0034] Take 120g of Ca-1 catalyst, add 14.4g of zinc nitrate (Zn content 5.0wt%) solution for impregnation, and prepare a Zn-containing sample with an atomic ratio of Zn to Pt of 4:1, and then at a heating rate of 3°C / min, Raise the temperature to 250°C for 10 hours, and then continue to raise the temperature to 410°C for 3 hours at a rate of 3°C / min. The obtained catalyst is numbered Ea-1.
[0035] Add 8.5wt% (as HNO 3 Add 100 g of the above-mentioned catalyst Ea-1 to 300 g of nitric acid aqueous solution, soak at room temperature for 10 hours, and then dry at 120° C. for 10 h to obtain catalyst E-1 o...
Embodiment 2
[0037] The preparation of catalyst E-2 of the present invention
[0038] Take 120g of Ca-1 catalyst, add 15g of zinc acetate (Zn content 10.0wt%) solution for impregnation to prepare a Zn-containing sample, wherein the atomic ratio of Zn to Pt is 5:1, and then heat up at a rate of 3°C / min , heated to 230° C. for 10 hours, and then continued to heat up to 420° C. for 3 hours at a rate of 3° C. / min. The obtained catalyst was numbered Eb-1.
[0039] Add 7.5wt% (as HNO 3 Add 100 g of the above-mentioned catalyst Eb-1 to 300 g of nitric acid aqueous solution, soak at room temperature for 5 hours and then dry at 110° C. for 8 hours to obtain catalyst E-2 of the present invention.
Embodiment 3
[0041] The preparation of catalyst E-3 of the present invention
[0042] Add 1450ml of organic solvent (the volume ratio of gasoline and kerosene is 1:1) into a 2000ml flask to treat the deactivated catalyst FD-1 after industrial operation, and the catalyst number after 2 hours of distillation is Cb-1.
[0043] Take 120g of Cb-1 catalyst, add 72g of zinc nitrate (Zn content 2.0wt%) solution for impregnation to prepare a Zn-containing sample, wherein the atomic ratio of Zn to Pt is 8:1, and then heat up at a rate of 3°C / min , heated to 280°C for 8 hours, and then continued to heat up to 350°C for 5 hours at a rate of 3°C / min. The obtained catalyst was numbered Ec-1.
[0044] Add 15% (as HNO 3 Add 100 g of the above-mentioned catalyst Ec-1 to 300 g of nitric acid aqueous solution, soak at room temperature for 10 hours, and then dry at 120° C. for 10 hours to obtain the catalyst E-3 of the present invention. Its physical and chemical properties are shown in Table 3, and the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com