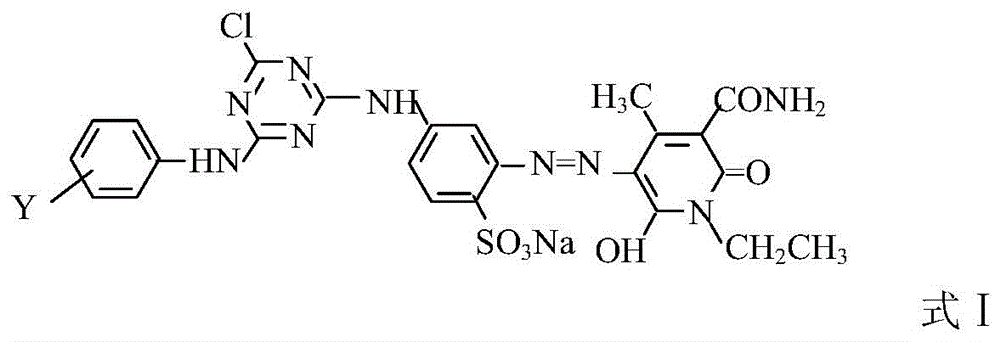
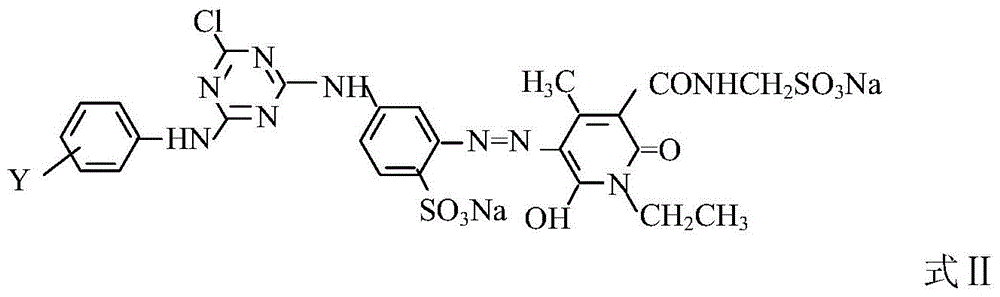
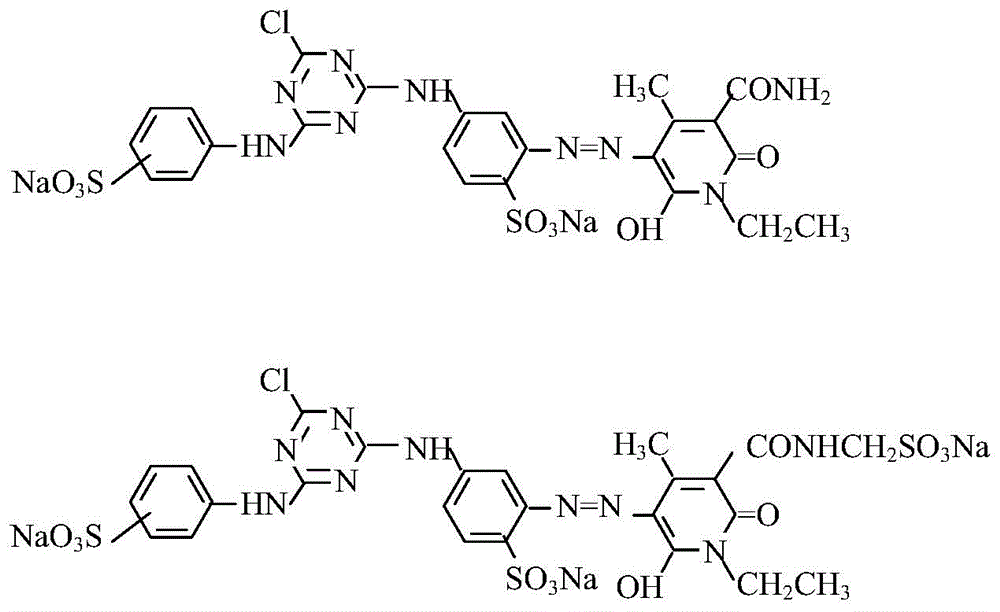
A kind of composite active light yellow dye composition
A dye composition and reactive bright yellow technology, applied in the field of dyes, can solve the problems of poor color fixation rate, unsuitable for automatic size mixing system, unfavorable for environmental protection, etc., achieve good printing lifting power, good controllability of shade, and dyeing improvement good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Beat 1.02 moles of cyanuric chloride with ice and water for 1 hour, control the speed for 1 hour, add 1 mole of 2,4-diaminobenzenesulfonic acid solution with a pH value of 6.5 to 7.0 and a concentration of 20%, and control the temperature at 0 to 5 Carry out a condensation reaction at ℃, V=700-750L / 0.1KM, quickly adjust the pH=7.0-7.5 with 15% soda ash after 2 hours, add 3.5 moles of hydrochloric acid, 1.0 moles of sodium nitrite solution for diazotization, and keep T=0 -10°C, starch KI test paper slightly blue, Congo red blue, after adding, keep the conditions, react for 1 hour, and use sulfamic acid to balance the excess nitrous acid.
[0022] 0.4 moles of N-ethyl-3-carbamoyl-4-methyl-6-hydroxy-2-pyridone and 0.6 moles of N-ethyl-3-sulfonylmethylcarbamoyl-4-methyl- 6-Hydroxy-2-pyridone is beaten with water, soda ash to adjust PH=6.0~7.0, after dissolving, add the above diazonium salt, soda ash to adjust PH=6.0~7.5, react at 5~10°C for 2~3 hours, measure diazo The dis...
Embodiment 2
[0024] In Example 1, N-ethyl-3-carbamoyl-4-methyl-6-hydroxyl-2-pyridone and N-ethyl-3-sulfonylmethylcarbamoyl-4-methyl- The molar ratio of 6-hydroxy-2-pyridone was changed to 0.5:0.5, the secondary condensate was changed to m-aminobenzenesulfonic acid, and other process conditions remained unchanged to obtain commercial dyes.
Embodiment 3
[0026] In Example 1, N-ethyl-3-carbamoyl-4-methyl-6-hydroxyl-2-pyridone and N-ethyl-3-sulfonylmethylcarbamoyl-4-methyl- The molar ratio of 6-hydroxy-2-pyridone was changed to 0.45:0.55, the secondary condensate was changed to m-aminobenzenesulfonic acid, and other process conditions remained unchanged to obtain commercial dyes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| color fastness | aaaaa | aaaaa |
| soaping fastness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com