Catalyst for synthesizing 2,6-dimethyl-4-heptanoneand preparation method for catalyst
A technology of diisobutyl ketone and catalyst is applied in the field of catalyst for synthesizing diisobutyl ketone and its preparation field, which can solve the problems of high cost of raw materials and only 14% selectivity of diisobutyl ketone, so as to improve the anti-coagulation resistance. Carbon capacity, good dehydration function, and the effect of improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
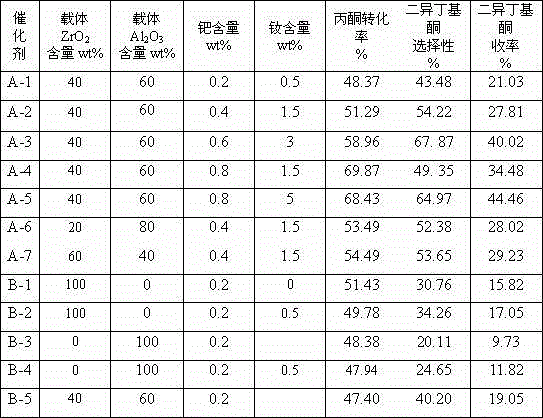
[0024] (1) Weigh ZrOCl 2 ·8H 2 O 145g, dissolved in 400mL distilled water to form an aqueous solution, 80g Al 2 o 3 The powder was added to this solution and diluted with 10 (v / v)% NH under vigorous stirring 3 ·H 2 O solution was titrated to pH 9, then stirred for 30 minutes, left for 4 hours to age the precipitate, and then washed with distilled water until Cl-free - until. After drying at 110°C, bake at 550°C for 4 hours to obtain Al 2 o 3 -ZrO 2 Composite carrier. ZrO 2 The content is 40wt%, Al 2 o 3 The content is 60wt%.
[0025] (2) 1.6gNd(NO 3 ) 3 4H 2 O was added to 105mL of distilled water to form an aqueous solution, and 100g of Al was impregnated with this solution 2 o 3 -ZrO 2 Composite carrier, impregnated for 24 hours. Pour off the upper layer of clear water, dry at 110°C for 3 hours, and then bake at 500°C for 6 hours to make the carrier C-1 containing neodymium.
[0026] (3) Add 0.51gPd(NO 3 ) 2 2H 2 O was dissolved in 105 mL of distilled ...
Embodiment 2
[0028] (1)Al 2 o 3 -ZrO 2 The preparation of the composite carrier is the same as step (1) of Example 1.
[0029] (2) 4.8gNd(NO 3 ) 3 4H 2 O was added to 105mL of distilled water to form an aqueous solution, and 100g of Al was impregnated with this solution 2 o 3 -ZrO 2 The composite carrier was impregnated for 24 hours. Pour off the upper layer of clear water, dry at 110°C for 3 hours, and then bake at 500°C for 6 hours to make the carrier C-2 containing neodymium.
[0030] (3) 1.02gPd(NO 3 ) 2 2H 2 O was dissolved in 105 mL of distilled water to make an aqueous solution, and the pH value was adjusted to 3-4. Immerse 100g of C-2 into the palladium solution, and bake it at 400°C for 8 hours after the water evaporates. Catalyst A-2 was prepared. The Nd content of the catalyst was 1.5 wt%, and the Pd content was 0.4 wt%.
Embodiment 3
[0032] (1)Al 2 o 3 -ZrO 2 The preparation of the composite carrier is the same as step (1) of Example 1.
[0033] (2) 9.6gNd(NO 3 ) 3 4H 2 O was added to 105mL of distilled water to form an aqueous solution, and 100g of Al was impregnated with this solution 2 o 3 -ZrO 2 Composite carrier, impregnated for 24 hours. Pour off the upper layer of clear water, dry at 110°C for 3 hours, and then bake at 500°C for 6 hours to make the carrier C-3 containing neodymium.
[0034] (3) 1.51gPd(NO 3 ) 2 2H 2 O was dissolved in 105 mL of distilled water to make an aqueous solution, and the pH value was adjusted to 3-4. Immerse 100g of C-3 into the palladium solution, and bake it at 400°C for 8 hours after the water evaporates. Catalyst A-3 was prepared. The Nd content of the catalyst is 3wt%, and the Pd content is 0.6wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com