Immunomodulatory agent and uses therefor
A technology for inhibitors and joint damage, applied in the field of immunomodulators and their uses, can solve problems such as proving citrulline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
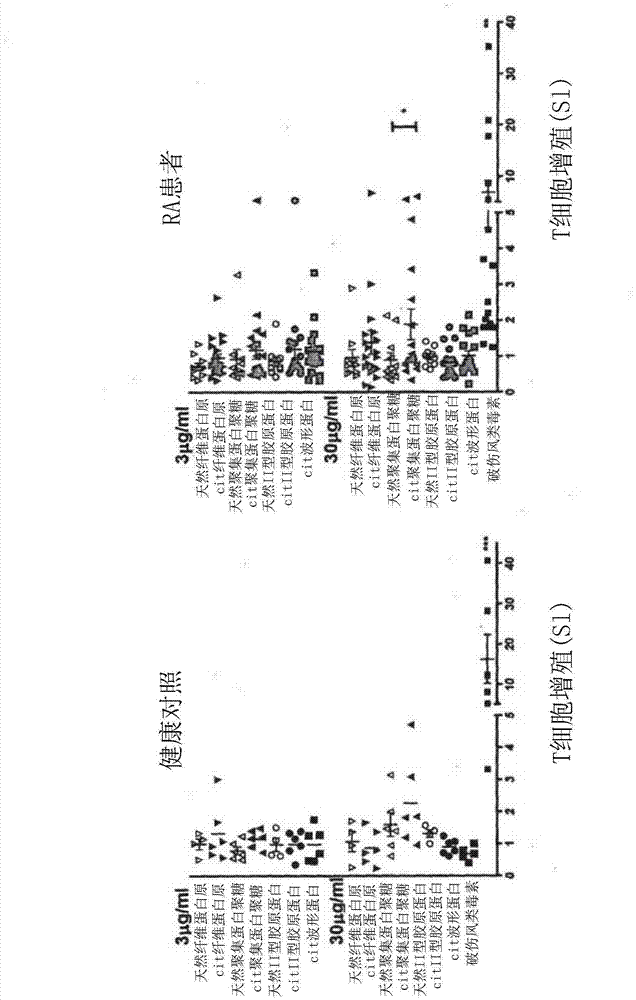
[0446] T cells proliferate inadequately but produce cytokines in response to citrullinated autoantigen peptides
[0447] Based on the predicted binding capacity to RA-associated DR molecules in molecular models of P4-localized citrulline, or through previous studies in HLA-DR4-IE-transgenic mice, established fibrinogen, vimentin, Synthesis of citrullinated or unmodified peptide antigens from collagen and aggrecan protein sequences (Table 5) (Hida et al., 2004.J.Autoimmun.23:141-150; Hill J et al., 2008.J.Exp.Med 205:967-979; von Delwig et al., 2010. Arthritis Rheum. 62:143-149).
[0448] Table 5. Sequences of the peptides used in the examples
[0449]
[0450] A total of 21 cases of SE were studied + RA patient and 6 SE + healthy controls. All but 4 RA patients were also ACPA +(Table 6). Forty-three percent of RA patients were non-smokers, 38% were past smokers, and 19% were current smokers. One healthy control is a current smoker, and 2 healthy controls have a famil...
Embodiment 2
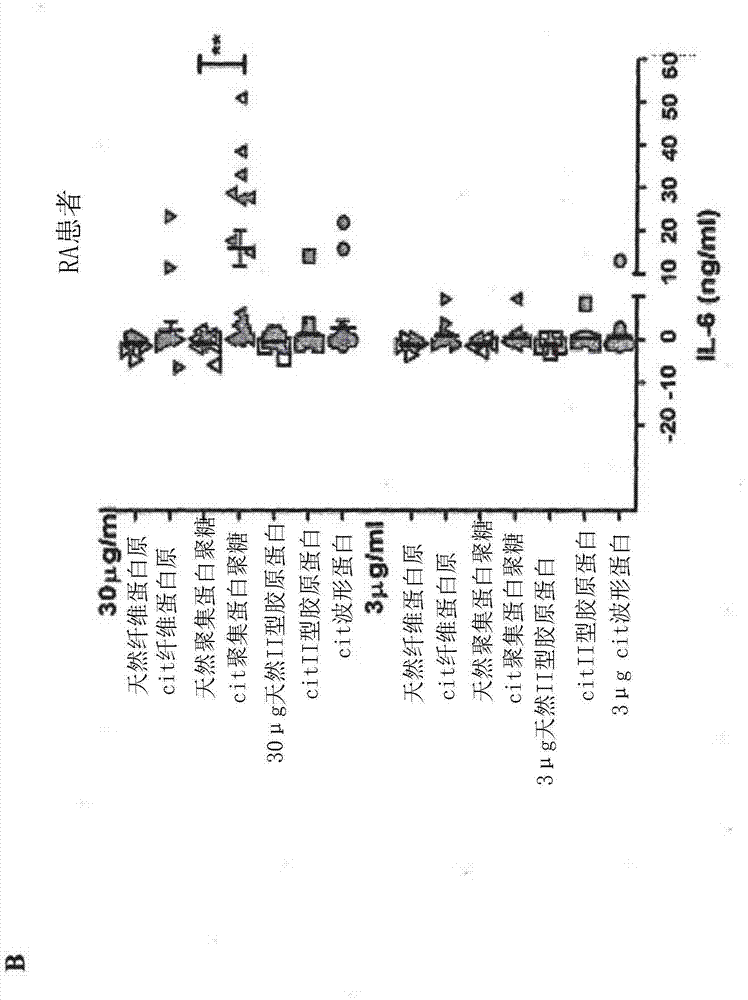
[0453] IL-6 response changes with disease duration in RA patients
[0454] To better understand individual RA patients and SE + Citrullinated Peptide Response Patterns of Healthy Controls, IL-6 dose response curves for each peptide were plotted for each individual in the study. PBMCs from 4 / 6 healthy controls secreted IL-6 in a dose-dependent manner in response to citrullinated aggrecan, and 3 / 6 secreted IL-6 in response to citrullinated fibrinogen. Using the same thresholds for positive responses as described above, it was found that of the 17 PBMC responses from RA patients, 6 secreted IL-6 in no response to the epitope, 8 responded only to citrullinated aggrecan, and 0 responded Citrullinated fibrinogen, 0 cases responded only to citrullinated type II collagen, and 3 cases responded to multiple citrullinated epitopes. therefore. IL-6 responses to citrullinated aggrecan were highest and most frequently observed in RA patients and healthy controls, suggesting that this was...
Embodiment 3
[0456] effector memory CD4 + T cells secrete cytokines in response to citrullinated peptides
[0457] IL-6 is an important cytokine in RA, which is produced when PMBCs are stimulated by T cells or antigen-presenting cells. To determine the source of cytokines secreted into the supernatants of citrullinated peptide-stimulated PBMCs, RA PBMCs were incubated with citrullinated or native peptides for 5 days prior to intracellular cytokine staining and analysis of cell surface markers , and Brefeldin-A was added for the last 18 hours. When incubated with citrullinated aggrecan or fibrinogen, CD3 + CD4 + T cells produce more intracellular IFN-γ and IL-6 ( Figure 6 A, B). Fluorescence compensated control (FMO) staining demonstrated a gating strategy to determine the threshold for positive staining as described (Herzenberg et al., 2006. Nat Immunol 7(7):681-685) ( Figure 6 C). CD4 - CD28 - In these assays performed on cells (the majority of which represent antigen-presentin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com