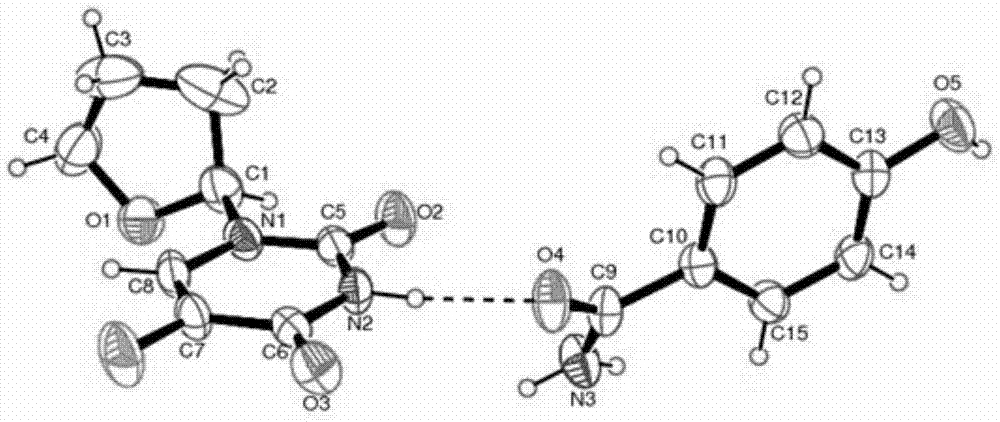
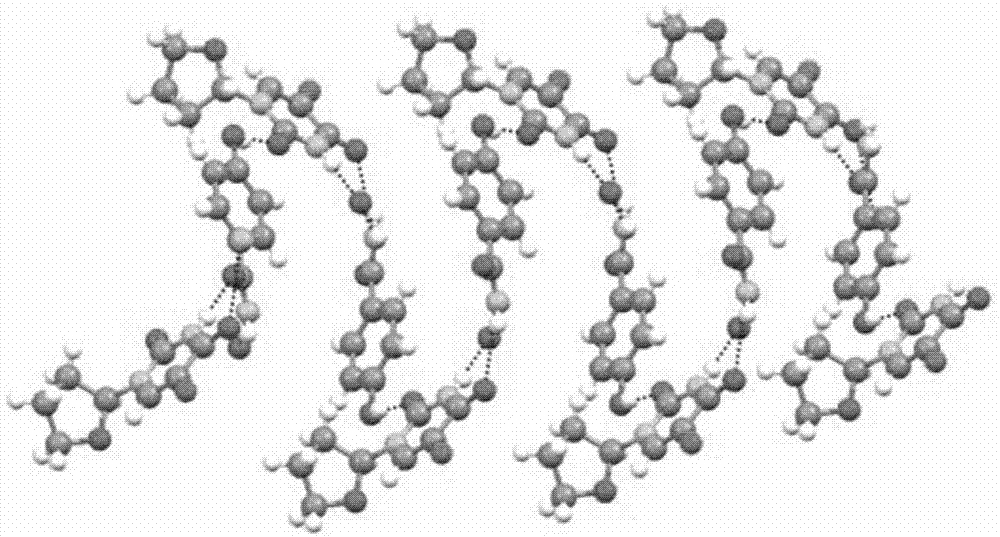
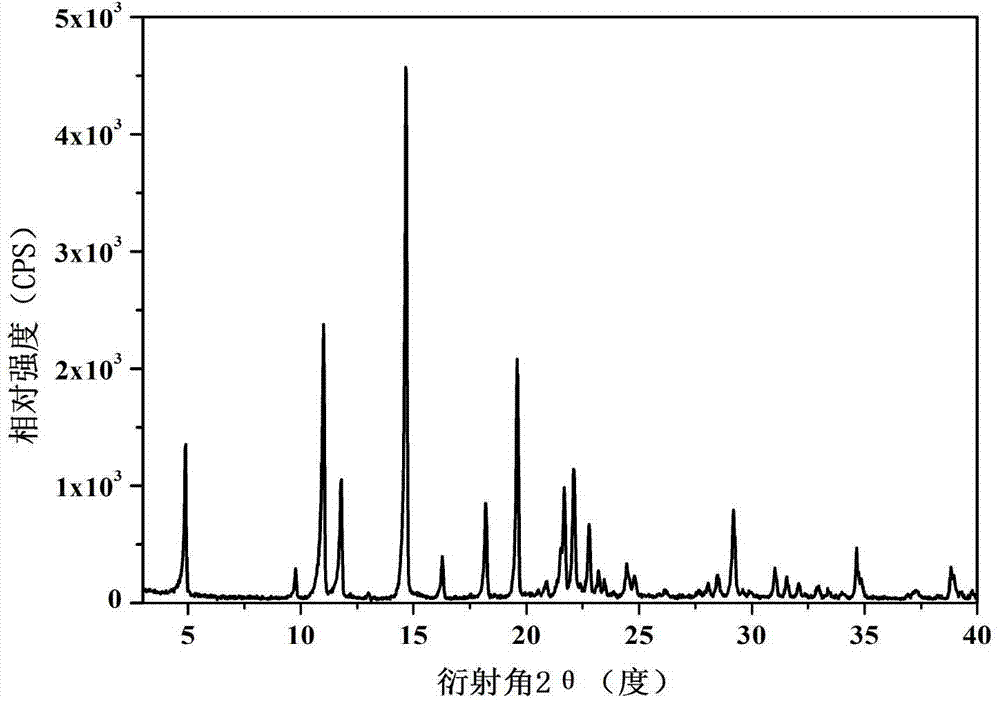
Novel pharmaceutical tegafur co-crystal and preparation method thereof
A technology of tegafur and drugs, which is applied in the field of preparation of new co-crystals and new co-crystals to achieve the effects of improved bioavailability, improved stability and improved thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take 0.40g of tegafur and 0.27g of 4-hydroxybenzamide, add 2ml of acetonitrile solution, heat to 60°C and reflux, stir to dissolve, continue to reflux for 20-30 minutes, quickly put it in an ice-water bath, and slowly precipitate white After 24 hours, the crystals were filtered to obtain a white tegafur organic drug co-crystal.
Embodiment 2
[0042] Take 0.40g of Tegafur and 0.27g of 4-hydroxybenzamide, add 4ml of acetonitrile solution, heat to reflux at 60°C, stir to dissolve, continue to reflux for 20-30 minutes, quickly put it in an ice-water bath, and slowly precipitate white After 24 hours, the crystals were filtered to obtain a white tegafur organic drug co-crystal.
Embodiment 3
[0044] Take 0.80g of Tegafur and 0.54g of 4-hydroxybenzamide, add 5ml of acetonitrile solution, heat to reflux at 60°C, stir to dissolve, continue to reflux for 20-30 minutes, quickly put it into an ice-water bath, and slowly precipitate white After 24 hours, the crystals were filtered to obtain a white tegafur organic drug co-crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com