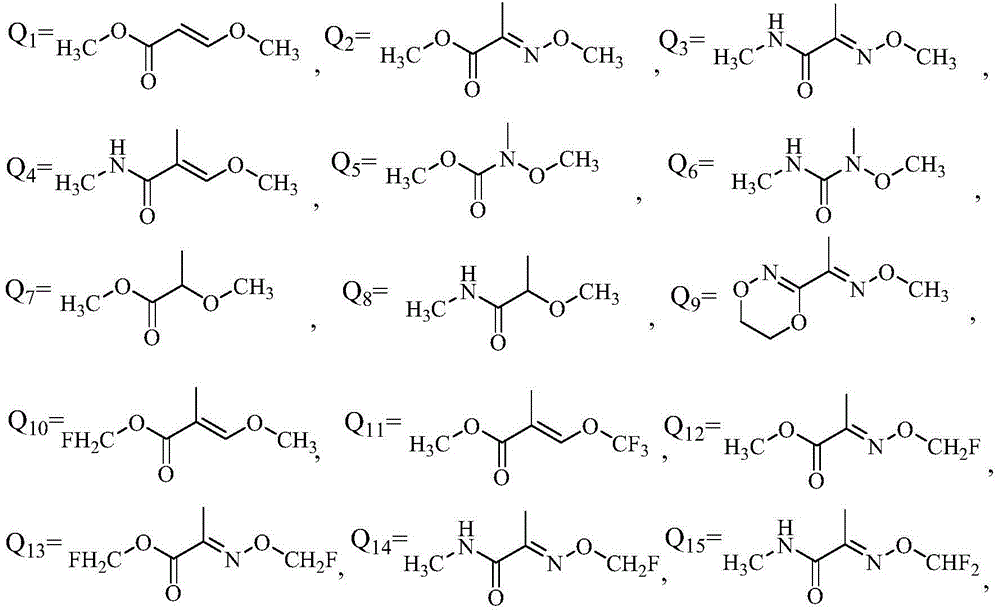
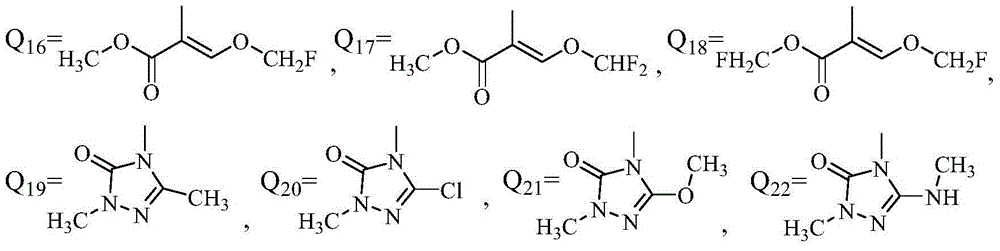
Application of phenyl ether compound with antitumor activity
A compound, phenyl ether technology, applied in the application field of phenyl ether compounds as anti-tumor drugs, can solve the problem of no reports of anti-tumor drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: For the growth inhibition rate of human bladder cancer cells J82, LNCap, T24, and prostate cancer cell PC-3, about 1000 to 3000 cells were planted in a 24-well plate using in vitro cell culture technology, and then each well was Add 1 ml of cell culture medium well known to those skilled in the art that can cultivate the tumor cell lines used in the test, and place in a cell culture incubator (CO 2 5%, 370°C) for 24 hours, then add an appropriate concentration of the above-mentioned substance to be tested into the wells, and note that the volume of the added solution does not exceed 0.5% of the total volume. Let the cells continue to grow in the cell culture incubator. After one week, aspirate the cell culture medium and wash once with cold 1 ml PBS. Then, fix with 1% formalin at room temperature for 10 minutes, and wash once with cold 1 ml PBS. Add 0.1% crystal violet to stain for 30 minutes. Crystal violet recycling. The stained cells were washed slowly ...
Embodiment 2
[0079] Example 2: For the growth inhibition rate of human leukemia HL-60 cells, the conventional MTT method was used to measure the inhibition rate of the test samples on the growth of each human cancer cell. The cells were taken out from the incubator, washed twice with PBS solution, digested with 0.25% trypsin solution, and a medium well-known to those skilled in the art capable of cultivating tumor cell lines for testing was added to stop the digestion of each cell line, and after centrifugation, Pipette to form a cell suspension and count under an inverted microscope. And use the medium for cultivating each cancer cell line to prepare the cells to a concentration of 5x10 4 / ml of cell suspension, add 100 μl of cells to each well of a 96-well plate, place it in 5% carbon dioxide, and incubate overnight in humidified air at 37°C, add the above-mentioned drugs to be tested diluted into different concentration gradients, and let it act for 48 hours. MTT was added, and after 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com