Method for preparing purine derivatives
A compound and reaction mixture technology, applied in the field of preparation of purine derivatives, can solve the problem of high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0030] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0031] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0032] In the present invention, g means gram, and mL means milliliter.
Embodiment 1
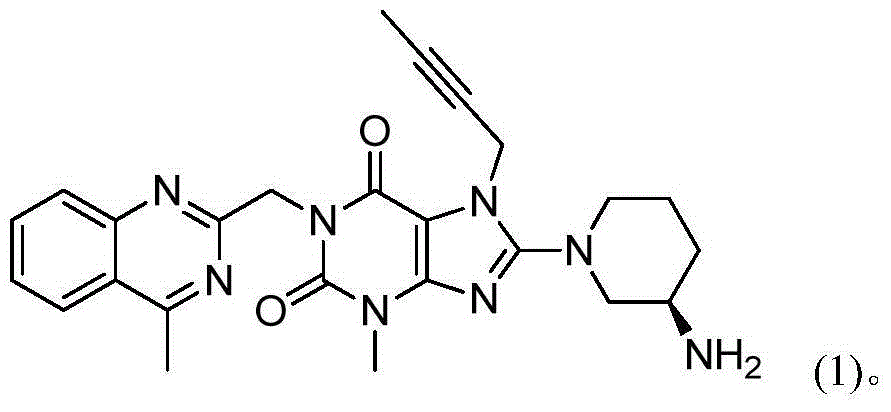
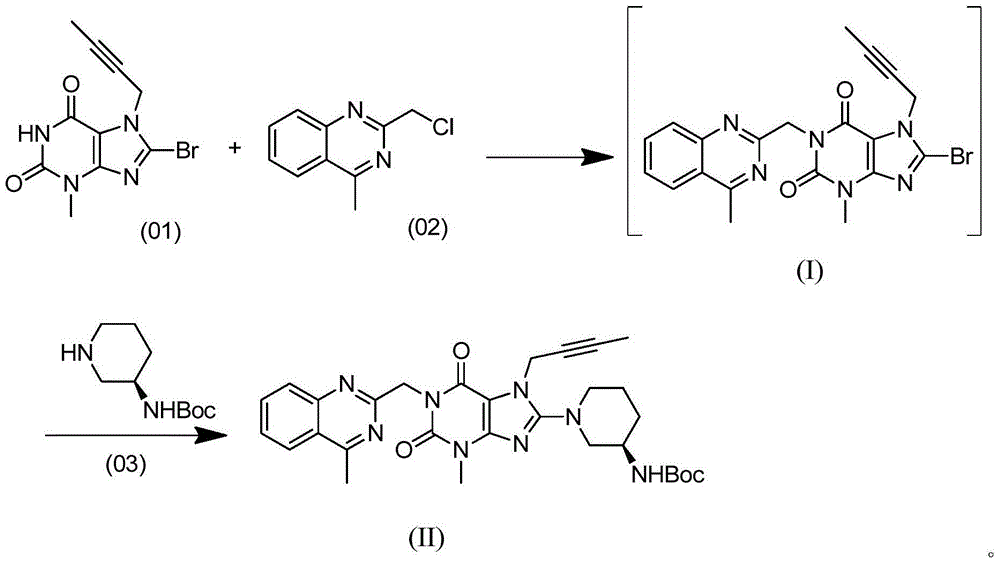
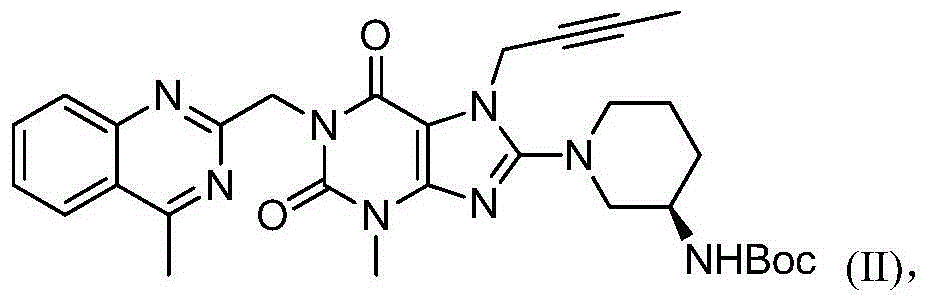
[0034] Add 150 mL of dimethyl sulfoxide, 10 g of compound (01), 7.13 g of compound (02), 9.30 g of potassium carbonate, and 0.61 g of tetrabutylammonium bromide into the reaction flask. The temperature was raised to 60° C. under stirring, and reacted for 6 hours to obtain the first reaction liquid. Add 8.2 g of compound (03) to the first reaction liquid, raise the temperature to 75°C-80°C and react for 16 hours, and the reaction is completed. 200mL of water was added dropwise to the reaction system, and after the dropwise addition, the temperature was lowered to 25°C and stirred for 2 hours; filtered, and the resulting solid was vacuum-dried at 45°C to obtain compound (II): 18.09g, purity: 97.5%.
Embodiment 2
[0036] Add 100 mL of N-methylpyrrolidone, 10.00 g of compound (01), 7.70 g of compound (02), 9.55 g of potassium carbonate, and 1.87 g of tetrabutylammonium chloride into the reaction flask. The temperature was raised to 65° C.-70° C. under stirring, and reacted for 4 hours to obtain the first reaction solution. Add 7.45 g of compound (03) to the first reaction liquid, raise the temperature to 80°C-85°C and react for 13 hours, and the reaction ends. Add 200mL of water dropwise to the reaction system. After the dropwise addition, cool down to 25°C and stir for 2h; filter and dry the obtained solid under vacuum at 45°C to obtain compound (II): 17.85g, purity: 97.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com