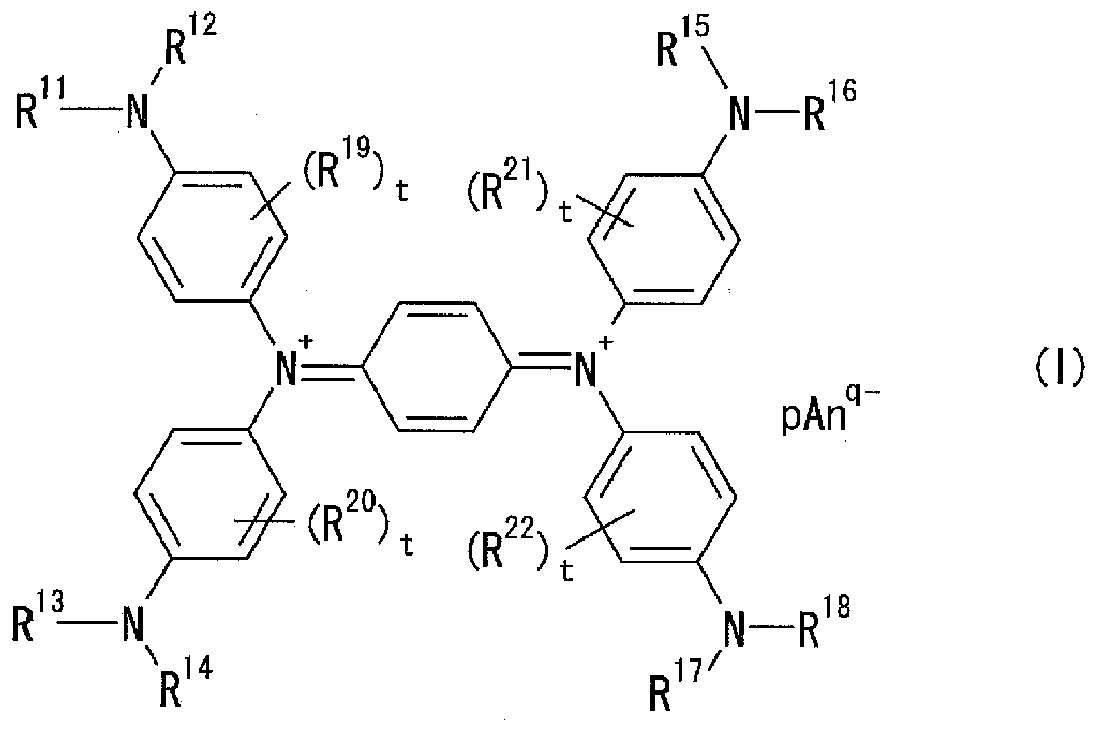
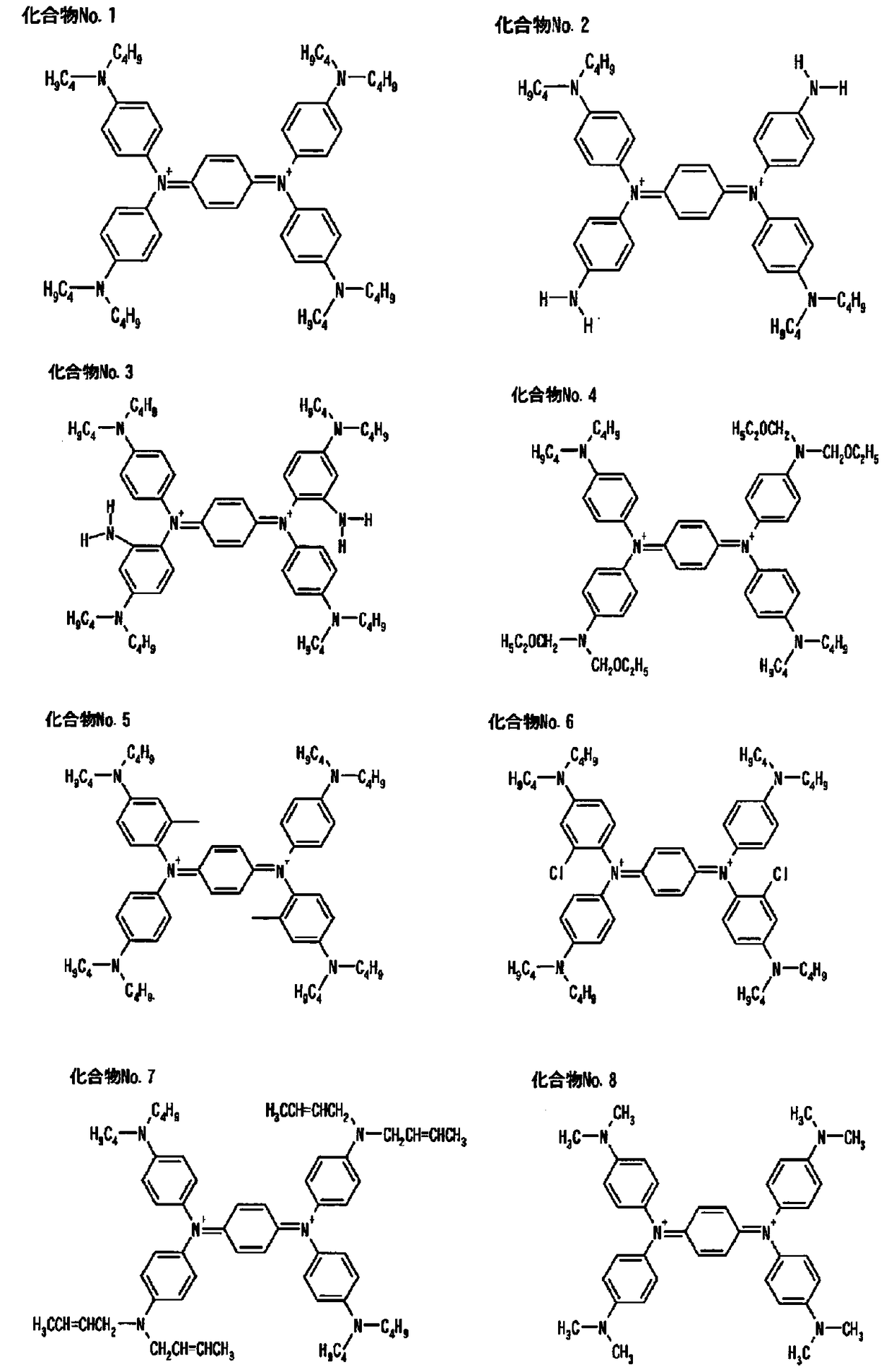
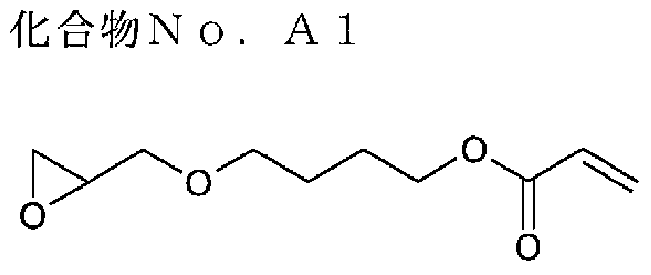
Coatings and near-infrared absorption filters
A near-infrared, infrared technology, applied in the direction of radiation-absorbing coatings, instruments, coatings, etc., can solve the problems of no hint, no record, etc., and achieve the effect of high light resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] [Example 1] Preparation of Coating No.1
[0062] 0.05 g of bis(trifluoromethanesulfonyl)imide salt of compound No. 1 as (A) component, and 2.75 g of diacetone alcohol as (C) component were added, stirred and dissolved. Then, 5 g of SPC-2000 (manufactured by Showa Denko Co., Ltd.) was mixed as (B) component, and it stirred until the insoluble matter disappeared, and paint No. 1 was obtained.
Embodiment 2~6 and comparative example 1~2
[0063] [Examples 2-6 and Comparative Examples 1-2] Preparation of Coating No.2-No.6 and Comparative Coating No.1-No.2
[0064] By the procedure similar to Example 1, each component was mixed with the formulation shown in Table 1, and paint No.2-No.6 and comparative paint No.1-No.2 were obtained.
[0065] Table 1
[0066]
[0067] *1: SPC-1000: Manufactured by Showa Denko
[0068] *2: Z-250: Made by Daicel Cytec
[0069] *3: Sumipex LG: Sumitomo Chemical Co., Ltd.
[0070] *4: DAA: diacetone alcohol
[0071] *5: DMAc: Dimethylacetamide
Embodiment 7~12 and comparative example 3~4
[0072] [Examples 7 to 12 and Comparative Examples 3 to 4] Production of near-infrared absorption filters No. 1 to No. 6 and comparison near-infrared absorption filters No. 1 to No. 2
[0073] On Lumirror U98, which is a PET film manufactured by Toray Co., Ltd., 2.5 mL of the coatings No. 1 to No. 6 obtained in Examples 1 to 6 and the comparative coatings No. 1 to No. 2 obtained in Comparative Examples 1 to 2 were dropped, Coating was done using a #14 rod coater. Thereafter, drying was performed at 100° C. for 15 minutes to manufacture near-infrared absorption filters No. 1 to No. 6 and comparative near-infrared absorption filters No. 1 to No. 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com