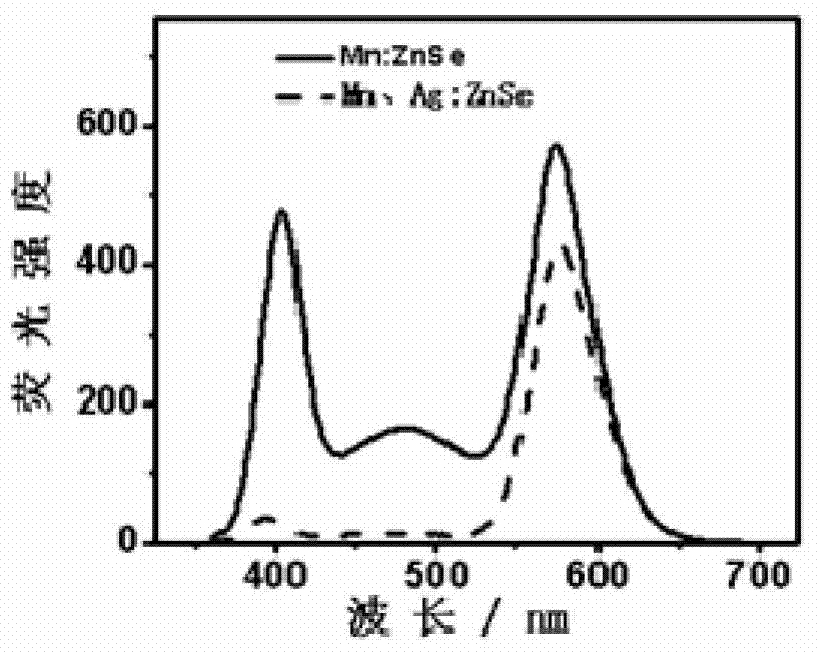
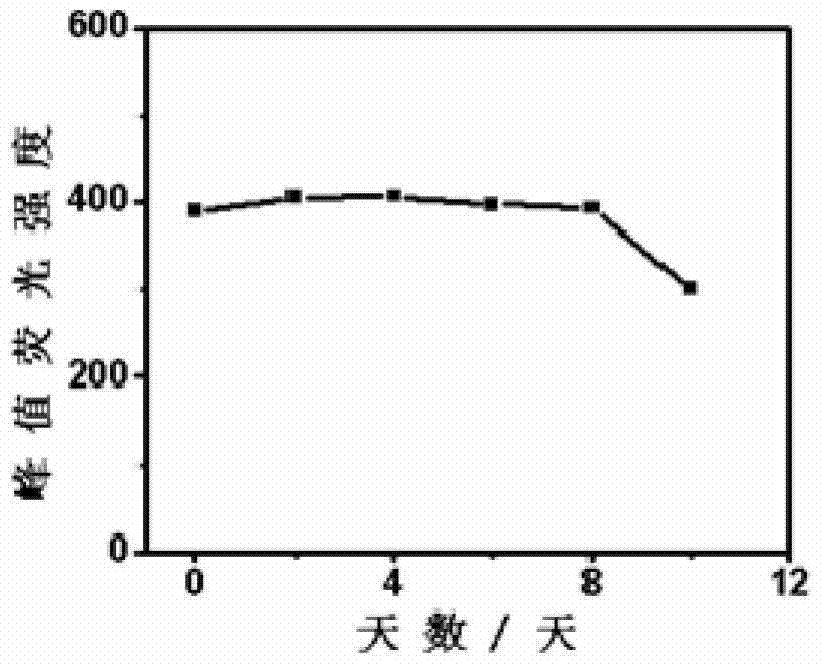
Preparation method of pure yellow fluorescence water-soluble doped zinc selenide quantum dots
A yellow fluorescent, water-soluble technology, applied in chemical instruments and methods, luminescent materials, etc., to achieve good water solubility, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] A preparation method of pure yellow fluorescent water-soluble doped zinc selenide quantum dots, comprising the steps of:
[0021] Step 1. Add 0.1mol / LMnCl to deionized water 2 Solution, 0.1mol / LAgNO 3 The solution and the ligand (mercaptoacetic acid or mercaptopropionic acid) containing mercapto groups are mixed evenly, and the pH is adjusted to 7.1 to 12.5, preferably 11.9, with 5mol / L sodium hydroxide solution to obtain an aqueous solution of impurity ion precursor; wherein, Mn 2+ and Ag + The molar ratio of Mn is 1:0.5~2, preferably 1:1.5, Mn 2+ The molar ratio to the ligand containing thiol is 1:20-50, preferably 1:37.3, Mn 2+ The concentration is 4×10 -5 mol / L. The solution cannot be left for a long time and should be used immediately after preparation.
[0022] Step 2. Under the ice bath, deoxygenate the impurity ion precursor aqueous solution prepared in step 1 with nitrogen gas, and the deoxygenation time is not less than 10 minutes. After that, NaHSe aque...
Embodiment 1
[0029] 1. Add 0.1mol / L MnCl to 200mL deionized water 2 Solution 80uL, 0.1mol / L AgNO 3 120 uL of the solution, followed by 26 uL of mercaptopropionic acid, stirred for 1 minute and then adjusted the pH of the mixed solution to 11.9 with 5 mol / L NaOH solution to obtain an aqueous solution of impurity ion precursor. This solution cannot be left for a long time, and it should be used immediately after preparation.
[0030] 2. Add the above-mentioned newly prepared impurity ion precursor solution into a round bottom flask with a capacity of 250mL, place the round bottom flask in an ice-water bath to cool for 30min, and deoxygenate the solution with high-purity nitrogen at the same time. After the temperature of the solution was cooled to 0°C, 0.25 mL of NaHSe aqueous solution with a concentration of 0.667 mol / L was quickly extracted with a syringe and injected into the above-mentioned flask under rapid stirring, and stirred for 5 min.
[0031] 3. Transfer the above-mentioned roun...
Embodiment 2
[0036] 1. Add 0.1mol / L MnCl to 200mL deionized water 2 Solution 80uL, 0.1mol / L AgNO 3 40 uL of the solution, followed by injection of 14 uL of mercaptopropionic acid, and after stirring for 1 minute, the pH of the mixed solution was adjusted to 7.1 with 5 mol / L NaOH solution to obtain an aqueous solution of impurity ion precursor. This solution cannot be left for a long time, and it should be used immediately after preparation.
[0037] 2. Add the above-mentioned newly prepared impurity ion precursor solution into a round bottom flask with a capacity of 250mL, place the round bottom flask in an ice-water bath to cool for 30min, and deoxygenate the solution with high-purity nitrogen at the same time. After the temperature of the solution was cooled to 0°C, 0.073 mL of NaHSe aqueous solution with a concentration of 0.667 mol / L was quickly extracted with a syringe and injected into the above flask under rapid stirring, and stirred for 5 min.
[0038] 3. Transfer the above-menti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com