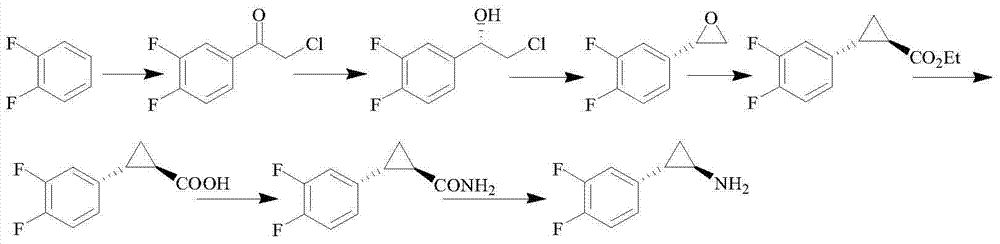
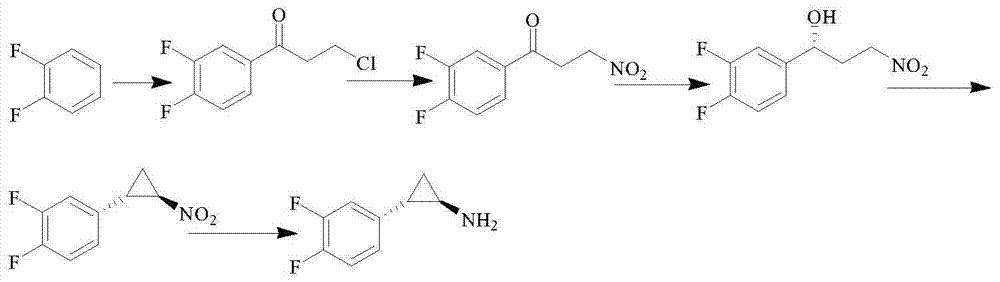
Method for preparing ticagrelor midbody (1R,2S)-2-(2,3-difluorophenyl) cyclopropylamine
A technology of difluorophenyl and ticagrelor, which is applied in the field of preparation of ticagrelor intermediate-2-cyclopropylamine, can solve the problems of unfavorable operator health and ecological environment, high cost of synthesis method and yield yield. Low efficiency and other problems, to achieve the effect of large-scale production, simple and convenient post-processing, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add compound I succinic anhydride (100 g, 1 mol) and ethanol (200 ml) into a reaction flask, reflux at 80-82° C. for 3 hours, and the reaction is complete; concentrate ethanol to obtain compound II (121 g, yield: 83%).
[0040] Add compound II (116.8g, 0.8mol), methylene chloride (300ml) and thionyl chloride (104g, 0.88mol) into the reaction flask, N 2 N, N-dimethylformamide (0.8 g) was added under protection, the temperature was raised at 38° C. under reflux and stirred for 5 hours, the reaction was complete, and the dichloromethane was concentrated to obtain compound III (124 g, yield: 95%).
[0041]Aluminum trichloride (102g, 0.77mol) and dichloromethane (400ml) were added to the reaction flask, the temperature was lowered to 0-5°C under the protection of nitrogen, and compound III (114.8g, 0.7mol) and o-difluorobenzene ( 80g, 0.7mol) mixed solution, the temperature of the whole process is less than 10°C, after dropping, keep stirring for 6 hours, then naturally rise...
Embodiment 2
[0047] Take compound I succinic anhydride (100g, 1mol) and isopropanol (250ml) into the reaction flask, heat and reflux at 82-85°C for 4 hours, and the reaction is complete; concentrate the isopropanol to obtain compound II (107g, yield: 67 %).
[0048] Add compound II (96g, 0.6mol), dichloromethane (200ml) and thionyl chloride (79g, 0.66mol) into the reaction flask, N 2 N,N-dimethylformamide (0.6g) was added under protection, heated to 42°C and refluxed to stir for 7 hours. After the reaction was complete, dichloromethane was concentrated to obtain compound III (102g, yield: 95.5%).
[0049] Aluminum trichloride (74g, 0.55mol) and dichloromethane (250ml) were added into the reaction flask, the temperature was lowered to 0-5°C under the protection of nitrogen, and compound III (89g, 0.5mol) and o-difluorobenzene (57g , 0.5mol) mixed solution, the whole temperature is less than 10°C, after dripping, keep stirring for 6 hours, then naturally rise to 28°C and react for 22 hours;...
Embodiment 3
[0055] Get compound I succinic anhydride (100g, 1mol) and n-butanol (300ml) into the reaction flask, heat and reflux at 119~112°C for 3.4 hours, the reaction is complete; concentrate n-butanol to obtain compound II (59g, yield: 34% ).
[0056] Add compound II (53g, 0.3mol), dichloromethane (200ml) and thionyl chloride (39g, 0.33mol) into the reaction flask, N 2 N, N-dimethylformamide (0.4 g) was added under protection, and the temperature was raised to 42° C. and refluxed and stirred for 6 hours. After the reaction was complete, the dichloromethane was concentrated to obtain compound III (52 g, yield: 91%).
[0057] Aluminum trichloride (30g, 0.22mol) and dichloromethane (100ml) were added to the reaction flask, the temperature was lowered to 0-5°C under the protection of nitrogen, and compound III (38g, 0.2mol) and o-difluorobenzene (23g , 0.2mol) mixed solution, the whole temperature is less than 10°C, after dripping, keep stirring for 6 hours and then naturally rise to 28°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com