Trisphenolyl methanes, production method and use thereof
A technology of trisphenol-based methane and a manufacturing method, which is applied in the directions of organic chemistry methods, chemical instruments and methods, preparation of hydroxy compounds, etc., can solve the problems of limited use and darkening of color tone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
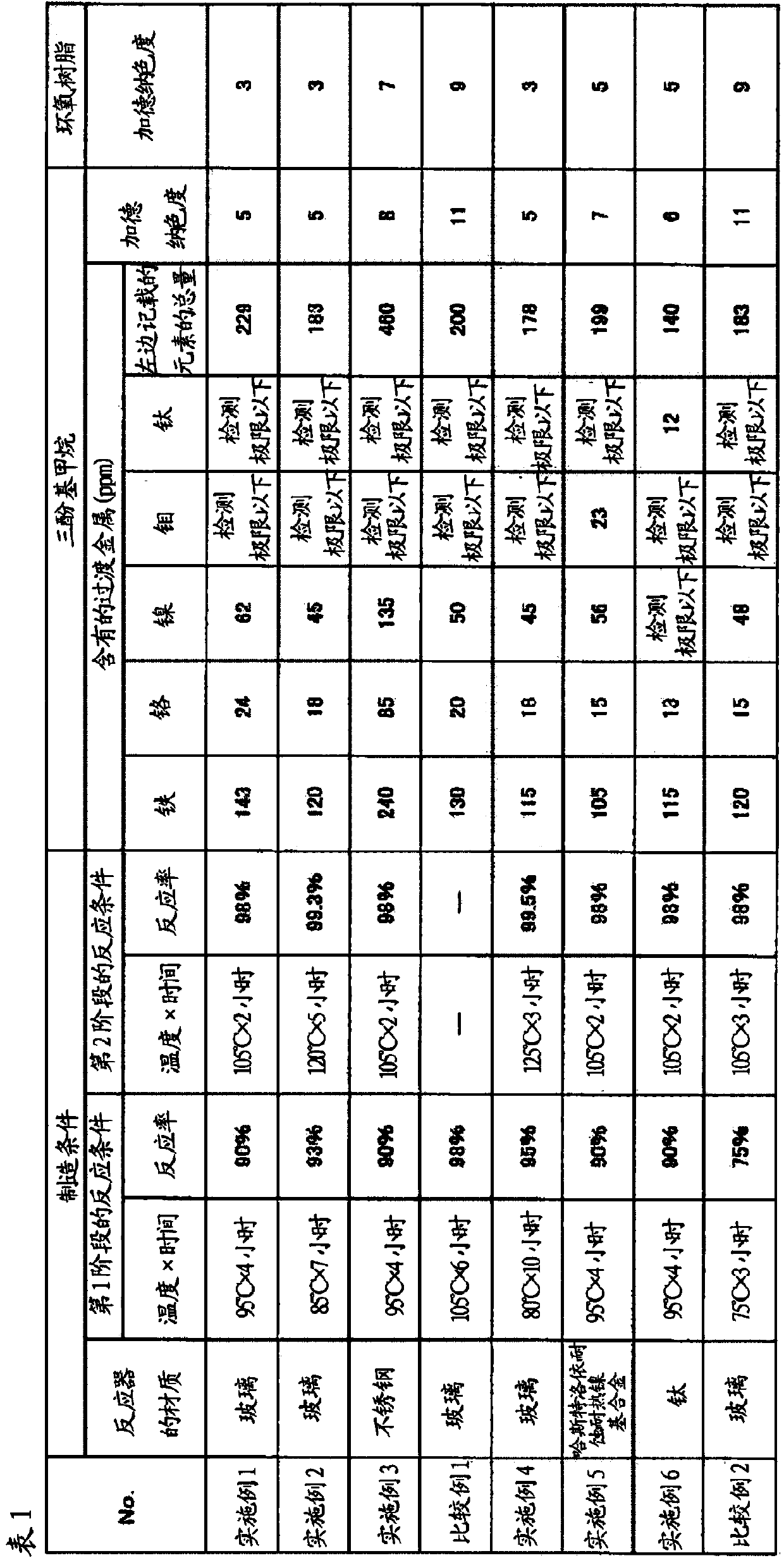
[0052]
[0053] 659 g (7 mol) of phenol, 61 g (0.5 mol) of salicylaldehyde, and para 1.425 g of toluenesulfonic acid (1.5 mol% relative to salicylaldehyde).
[0054] While bubbling nitrogen gas through an inert gas introduction tube, the reaction temperature was raised to 95° C. and reacted for 4 hours. According to the measurement result of salicylaldehyde measured by GPC, the reaction rate at this time was 90%.
[0055] Then, the reaction temperature was raised to 105° C. and allowed to react for 2 hours. The response rate was 98%.
[0056] After the reaction was completed, the temperature was lowered to 80° C., and 0.315 g of lithium hydroxide hydrate (1 equivalent to the catalyst) was added to neutralize the catalyst. Next, the contents were transferred to a 1-liter stainless steel reaction device, a distillation device was installed, and the reaction temperature was raised to 170° C. to remove unreacted phenol. Then, the pressure was reduced to 20 torr with a vacuum ...
Embodiment 2
[0062] In Example 1, the reaction temperature was raised to 85°C and allowed to react for 7 hours (reaction rate: 93%), then, the reaction temperature was raised to 120°C and allowed to react for 5 hours (reaction rate: 99.3%), Except for this, the same operation as in Example 1 was performed to obtain trisphenolomethanes and an epoxy resin.
[0063] Table 1 shows the metal content and Gardner color of the trisphenol methanes and the Gardner color of the epoxy resin.
Embodiment 3
[0065] In Example 1, except having replaced the reaction container with the reactor made of stainless steel, it carried out similarly to Example 1, and obtained trisphenol methanes and an epoxy resin.
[0066] Table 1 shows the metal content and Gardner color of the trisphenol methanes and the Gardner color of the epoxy resin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com