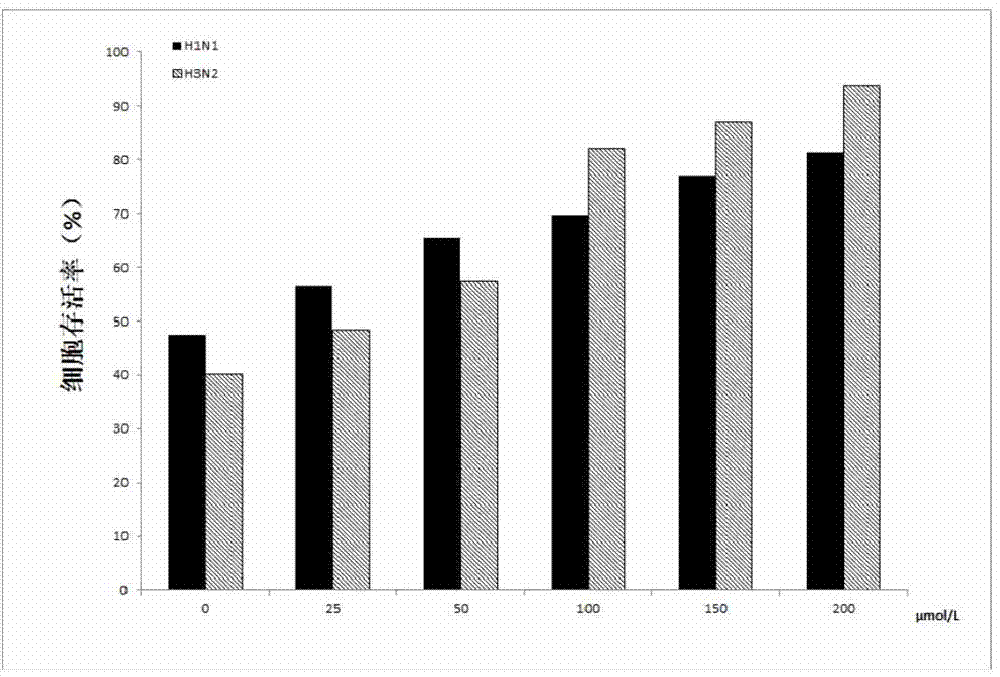Application of jatrorrhizine hydrochloride in preparation of drug for preventing and treating influenza
A technology of jatrorrhizine hydrochloride and medicine is applied in the application field of jatrorrhizine hydrochloride in the preparation of influenza virus-inhibiting medicines, and can solve the problems of unclear action principle, unknown effective ingredients, and hindered development of traditional Chinese medicines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
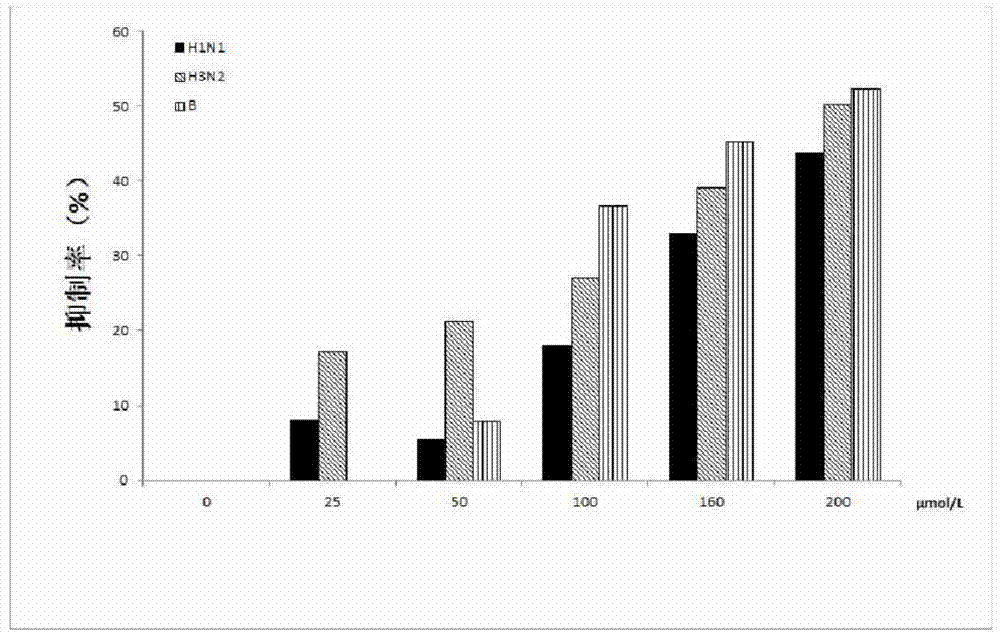
[0013] We used allantoic fluid containing influenza virus NA (passaged through chicken embryos, collected allantoic fluid, and stored at -80°C) to prepare in pH 6.5 MES buffer (32.5mM). A mixture of 41 μL jatrorrhizine hydrochloride was added to the reaction buffer, placed in a 37°C incubator for 30 minutes, then 50 μL of 20 μM MU-NANA substrate buffer was added, and detected on a microplate reader with an excitation wavelength of 360 nm. The emission wavelength is 450nm, and the detection time is 8min. The detection result is that jatrorrhizine hydrochloride is to H1N1, H3N2 and B type influenza virus NA half constant concentration (IC 50 ) were 120.40±20.07 μmol / L, 193.40±21.70 μmol / L and 178.05±8.57 μmol / L, respectively.
Embodiment 2
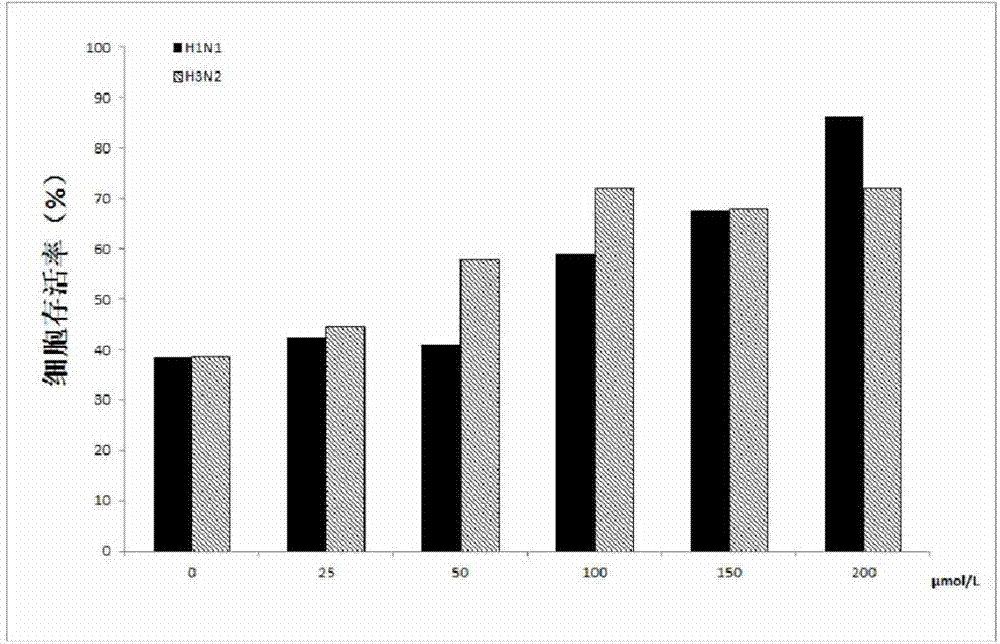
[0015] In order to detect the anti-influenza effect of jatrorrhizine hydrochloride at the cellular level, we experimented with two different treatments: first, the jatrorrhizine hydrochloride was diluted into different concentrations according to the concentration gradient, and the above solution was mixed with 100TCID 50 Equal volumes of different subtypes of influenza viruses (H1N1 and H3N2) were incubated for 2 hours in a total volume of 200 μL. Then add the mixture to MDCK cells and incubate for 2h; II add 100TCID 50 Different subtypes of influenza viruses (H1N1 and H3N2) were incubated in MDCK cells for 2 hours, and then incubated with different concentrations of jatrorrhizine hydrochloride for 2 hours. The maintenance solution (10 μg / ml trypsin in DMEM) was used for both different treatment dilutions. After 2 hours, the above-mentioned maintenance solution was changed once to continue culturing for 24 hours. After 24 hours, the maintenance solution containing 2% serum ...
Embodiment 3
[0018] In order to detect the protective effect of jatrorrhizine hydrochloride in the mouse influenza model, we used 50μL 10LD 50 Intranasally infected mice with FM1 virus. The experimental mice were randomly divided into 6 groups, namely the NC group; 10LD 50 group; 10LD50+50mg / kg Tamiflu group; jatrorrhizine hydrochloride low, medium and high dose groups: 10LD 50 +50, 100, 200mg / kg jatrorrhizine hydrochloride. There were 10 mice in each group, and the drug was given 1 day before the virus challenge, and the drug was administered by gavage, twice a day, for 7 consecutive days, and each mouse was gavaged with 0.2ml. The survival status and body weight changes of the mice were recorded every day. Then at day 15, the mice were sacrificed, the lung weight was recorded, the lung tissue was ground and added with PBS, and then the NA activity was measured.
[0019] The result is as Figure 4 and Figure 5 As shown, it shows that jatrorrhizine hydrochloride can inhibit the weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com