Improved method for preparation of inoculum suspension for ino-inositol test
A technology of bacterial suspension and inositol, which is applied in biochemical equipment and methods, microorganism-based methods, and microbial determination/inspection, etc., can solve problems such as inability to complete, poor correlation, and low absorbance, and achieve good linear correlation. , the effect of high accuracy and large correlation coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
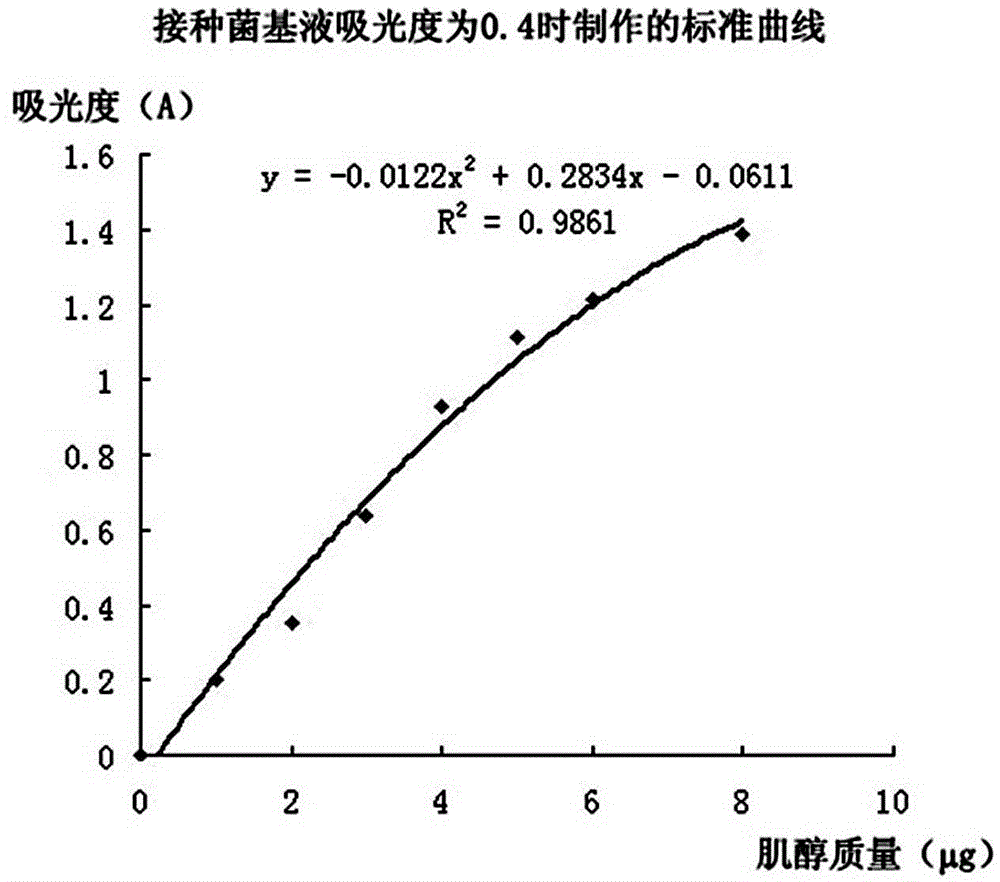
[0024] refer to figure 1 A method for detecting the content of inositol in food by microorganisms, comprising the following steps.
[0025] Step A, resuscitating the myo-inositol inoculum to obtain the resuscitated strain.
[0026] The inositol inoculum is grape juice yeast, which is activated and inoculated on the slant medium of malt extract powder agar, cultured at 30° C. for 16 hours, and then transferred for 2-3 generations to obtain the revived strain.
[0027] Step B, preparing the inoculum base solution.
[0028] Inoculate the resuscitated strain of step A into the inositol assay medium and continue to cultivate. After culturing at 30°C for 16 hours, the inoculum base solution is obtained. The inositol assay culture medium is 5 mL of inositol test medium and 5 mL of water. For the composition and preparation method of the inositol test medium, refer to the national food safety standard GB5413.25-2010 "Determination of inositol in food and dairy products for infants a...
Embodiment 2
[0065] The detection method of the present embodiment is different from embodiment 1 in that:
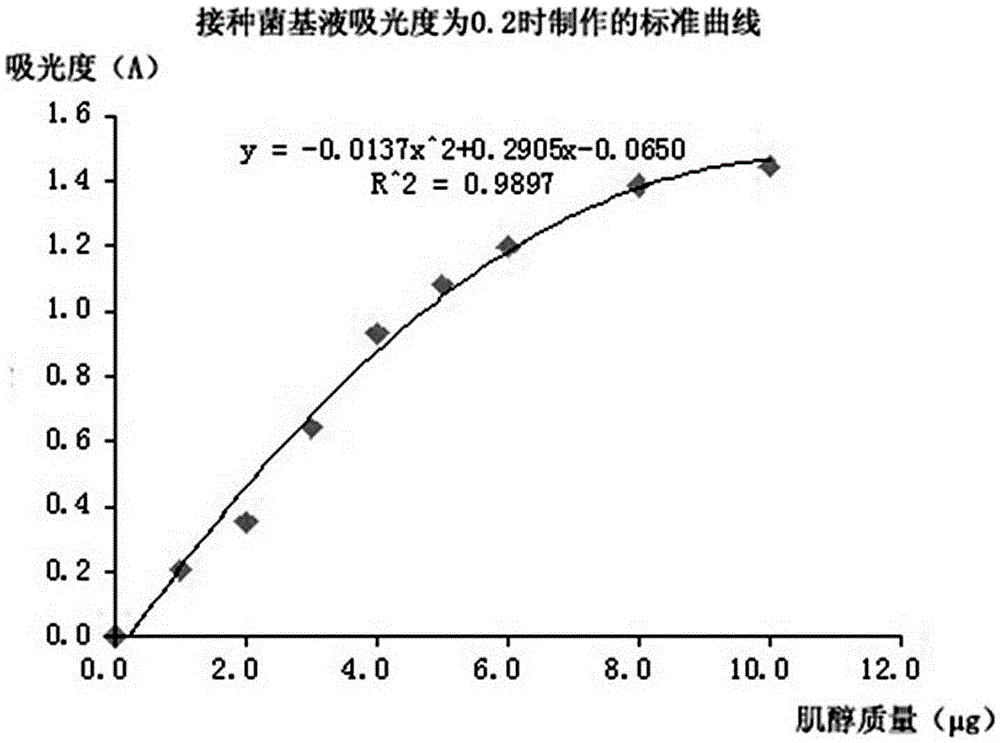
[0066] The absorbance of the inoculum base solution detected in step C is 0.2, and the inoculum base solution with an absorbance of 0.2 is taken to proceed to subsequent steps D and E.
[0067] Step E1, the standard curve made see image 3 .
[0068] Step E2. Weigh 2 samples, sample 1 has a mass of 2.0563 g, and sample 2 has a mass of 2.0095 g, and measure the absorbance of the liquid to be tested. The results are shown in Table 7.
[0069] Step E3, according to the absorbance of the liquid to be tested in Table 7, from image 3 The concentration of myo-inositol in the test solution was found in the shown standard curve, and the results are shown in Table 7. The calculated content of myo-inositol in the sample was 38.7mg / 100g.
[0070] Sample 1: m=2.0563g, Sample 2: 2.0095g,
[0071]
[0072] In order to test the reliability of the detection, the accuracy of the standard curv...
Embodiment 3
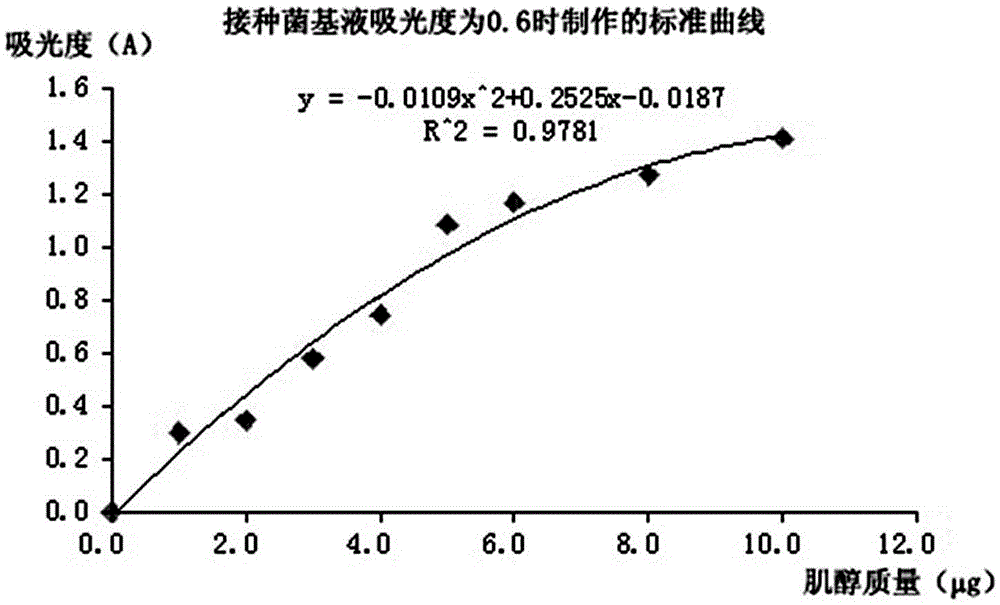
[0088] When the absorbance of the inoculum base liquid obtained after step A to step C of Example 180%, which is far from meeting the range of McFarland turbidity of 0.55-0.65, and the light transmittance required by the national standard In the range of 60%-80%, the concentration of the inoculated strain cannot meet the requirements of the test, and the inositol in the sample cannot be fully utilized, and accurate quantification cannot be performed. Therefore, the inoculum base solution with absorbance < 0.2 was discarded.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com