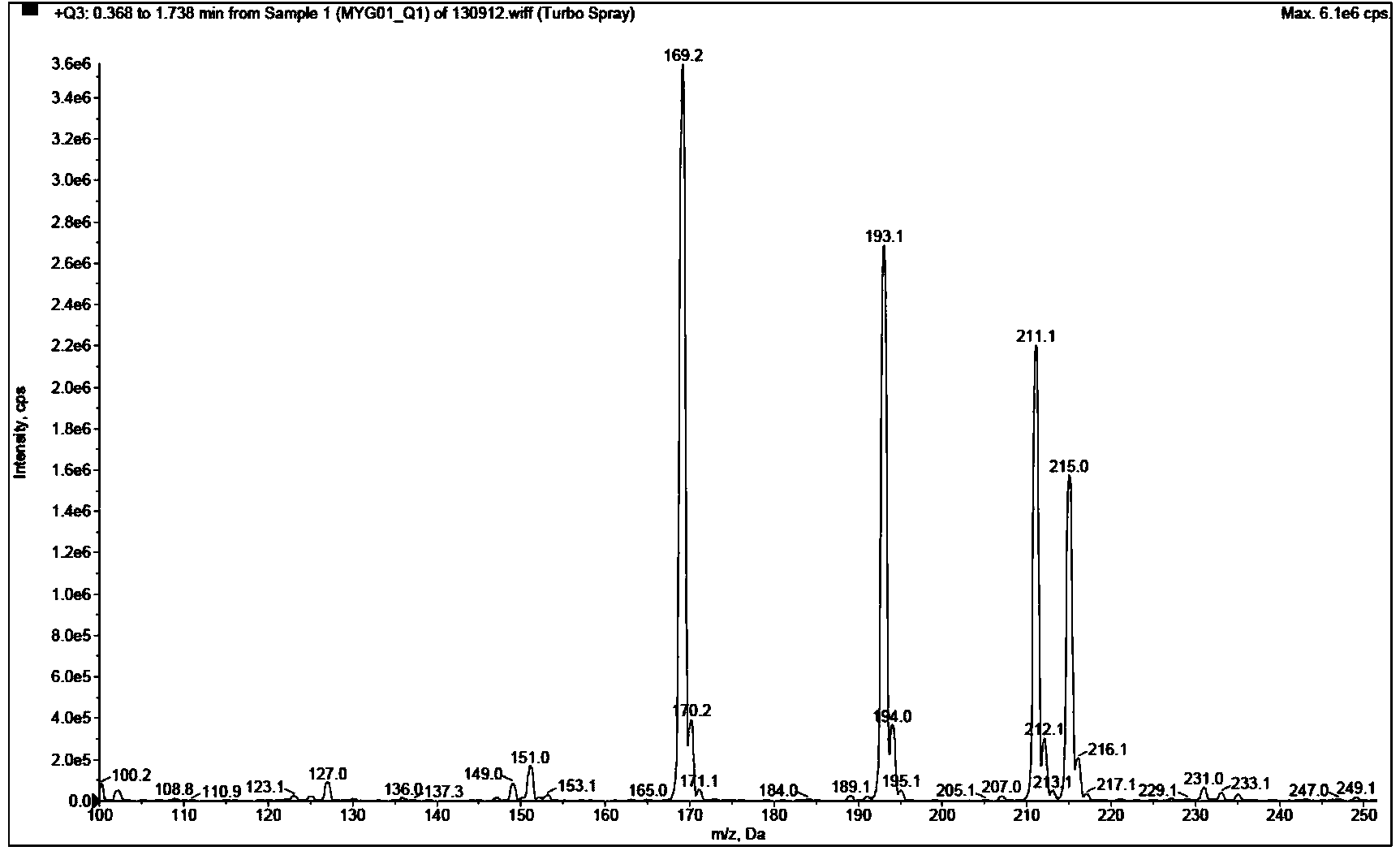
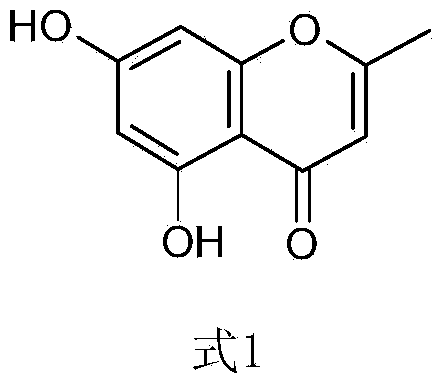
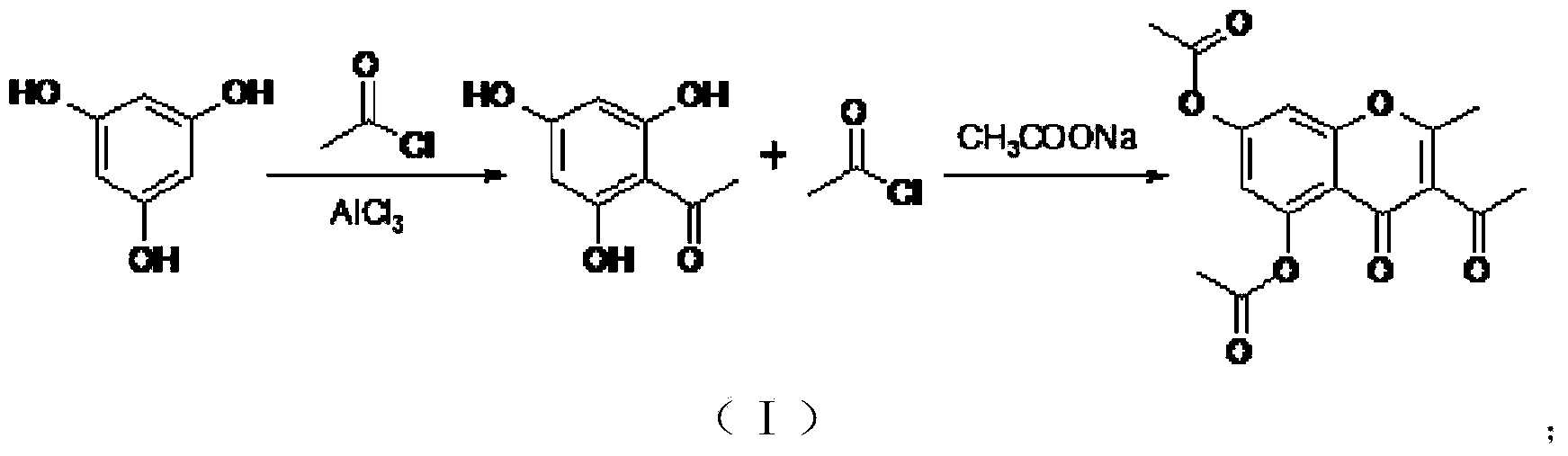
Chemical preparation method of compound 2-methyl-5,7-dihydroxy chromone
A technology of dihydroxychromone and compound is applied in the field of synthesis of chromone compound, which can solve the problems of easy generation of by-products and high cost of production raw materials, and achieves the effects of low cost, high cost and easy cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] "One-pot reaction" for the preparation of 2-methyl-3-acetyl-5,7-diacetoxychromone
[0054] (1) Acylation-cyclization:
[0055] Phloroglucinol raw material (12.6 g, 0.1 mol) was added into a 1 L three-necked flask, and 100 mL of dichloromethane was added to dissolve it. Under the condition of stirring at -15°C, anhydrous aluminum trichloride (16g, 0.12mol) catalyst was added, and acetyl chloride (8.6g, 0.11mol) was added dropwise. After the dropwise addition was completed, the reaction was stirred at room temperature for 3 hours. After the reaction was monitored by TLC and the reaction was complete, that is, after the raw material phloroglucinol was completely consumed and converted, anhydrous sodium acetate (28.7g, 0.35mol) was added to the reaction solution; and acetyl chloride (78g, 1.0mol) was added dropwise, The dropwise addition was completed within half an hour. The temperature was raised to reflux, and the reaction was stirred for 2 hours. After the reaction w...
Embodiment 2
[0064] "One-pot reaction" for the preparation of 2-methyl-3-acetyl-5,7-diacetoxychromone
[0065] (2) Acylation-cyclization:
[0066] Add phloroglucinol raw material (12.6 g, 0.1 mol) into a 1 L three-necked flask, and add 100 mL of 1,2-dichloroethane to dissolve it. At a temperature of 0°C and stirring, anhydrous aluminum trichloride (14 g, 0.105 mol) was added as a catalyst, and acetyl chloride (7.8 g, 0.1 mol) was added dropwise. After the addition was complete, the reaction was stirred at room temperature for 2 hours. After the reaction is monitored by TLC and the reaction is complete, that is, after the raw material phloroglucinol is completely consumed and converted, anhydrous sodium acetate (16.4g, 0.2mol) is added to the reaction solution; and acetyl chloride (63g, 0.8mol) is added dropwise, The dropwise addition was completed within half an hour. The temperature was raised to reflux, and the reaction was stirred for 1 hour. After the reaction, excess acetyl chlorid...
Embodiment 3
[0073] "One-pot reaction" for the preparation of 2-methyl-3-acetyl-5,7-diacetoxychromone
[0074] (1) Acylation-cyclization:
[0075] Add phloroglucinol raw material (12.6 g, 0.1 mol) into a 1 L three-necked flask, and add 100 mL of 1,2-dichloroethane to dissolve it. Under stirring conditions at a temperature of 10°C, anhydrous aluminum trichloride (16 g, 0.12 mol) was added as a catalyst, and acetyl chloride (9.4 g, 0.12 mol) was added dropwise. After the dropwise addition was completed, the reaction was stirred at room temperature for 5 hours. After the reaction was monitored by TLC and the reaction was complete, that is, after the raw material phloroglucinol was completely consumed and converted, anhydrous sodium acetate (41g, 0.5mol) was added to the reaction solution; and acetyl chloride (94g, 1.2mol) was added dropwise, and then The dropwise addition was completed within half an hour. The temperature was raised to reflux, and the reaction was stirred for 4 hours. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com