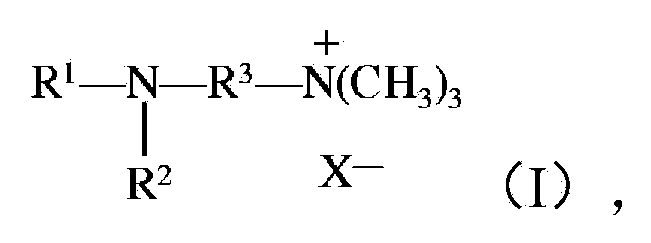Cleaning compositions comprising pH-switchable amine surfactants
A technology of cleaning composition and surfactant, applied in the field of cleaning composition, can solve the problems of reducing cleaning efficiency and effect, lack of water and other resources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0148] The following examples are provided by way of illustration only, and are not intended to limit the scope of the appended claims.
[0149] testing method :
[0150] pH Determination - Measure pH using a standard pH meter. It is believed that pH testing methods and devices are standardized so that those skilled in the art will know how to reliably determine the pH of a given solution. The pH meter is typically calibrated to the desired pH range (eg, pH 6 to pH 10) according to the manufacturer's instructions prior to use.
[0151] The pH should generally be measured at the actual use dilution as recommended by the detergent manufacturer. However, since such dilutions vary widely, the standard dilution herein is a detergent to water ratio of 1:350 by weight. The pH was obtained at 20°C. Unless specifically stated otherwise, the pH is measured neat.
[0152] Foam Test Protocol: Foam volume and foam mileage are measured by a FOAMSCAN instrument manufactured by Teclis ...
example 1
[0159] General Synthesis of pH-Switchable Cosurfactants
[0160] To a solution of 3-bromo-N,N,N-trimethylpropyl-1-ammonium bromide (119 g, 0.455 mol, 1.0 equivalent) in ethanol (1.2 L) at room temperature, add N-methyl Dodecane-1-amine (100 g, 0.501 mol, 1.5 equiv) in 100 mL ethanol. The resulting solution was refluxed for 18 h, then cooled to room temperature. Solid sodium hydroxide (18.2 g, 0.455 mol, 1.0 equiv) was added and the reaction was stirred at room temperature for 2 hours. The formed precipitate was vacuum filtered and the mother liquor was concentrated to afford a white solid. The solid was dissolved in water (300 mL) and washed with Et 2 O / EtOAc mixture (1:1 mixture, 3 x 300 mL) was extracted 3 times. The layers were separated and the aqueous layer was collected, frozen (-20°C freezer) and lyophilized in vacuo (48h). The resulting 3-(dodecyl(methyl)amino)-N,N,N-trimethylpropyl-1-ammonium bromide material was collected as a white fluffy solid (166 g).
[0...
example 2
[0186] Improved Synthesis of pH-Switchable Cosurfactants
[0187] To a solution of propylamine (5.5 mL, 0.067 mol) in EtOH (100 mL) was added portionwise 3-bromo-N,N,N-trimethylpropyl-1-ammonium bromide (5.0 g , 0.0191mol). The solution was warmed to room temperature, then refluxed for 4 hours. The solution was cooled to room temperature, and the solvent was removed via rotary evaporator to provide dibromo crude salt (5.69 g, 0.0179 mol). The salt was dissolved in MeOH (200 mL), and a solution of NaOH (0.717 g, 0.0179 mol) in MeOH (50 mL) was added portionwise at room temperature over 30 min. After the addition was complete, the solution was stirred at room temperature for an additional 2 hours, then the solvent was removed via a rotary evaporator. Combine the residue with CH 3 CN (150 mL) was stirred together for 30 minutes, and the solid was filtered off. The filtrate was concentrated via rotary evaporation and the residue was vacuum pumped for 24 h to provide 3-(prop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com