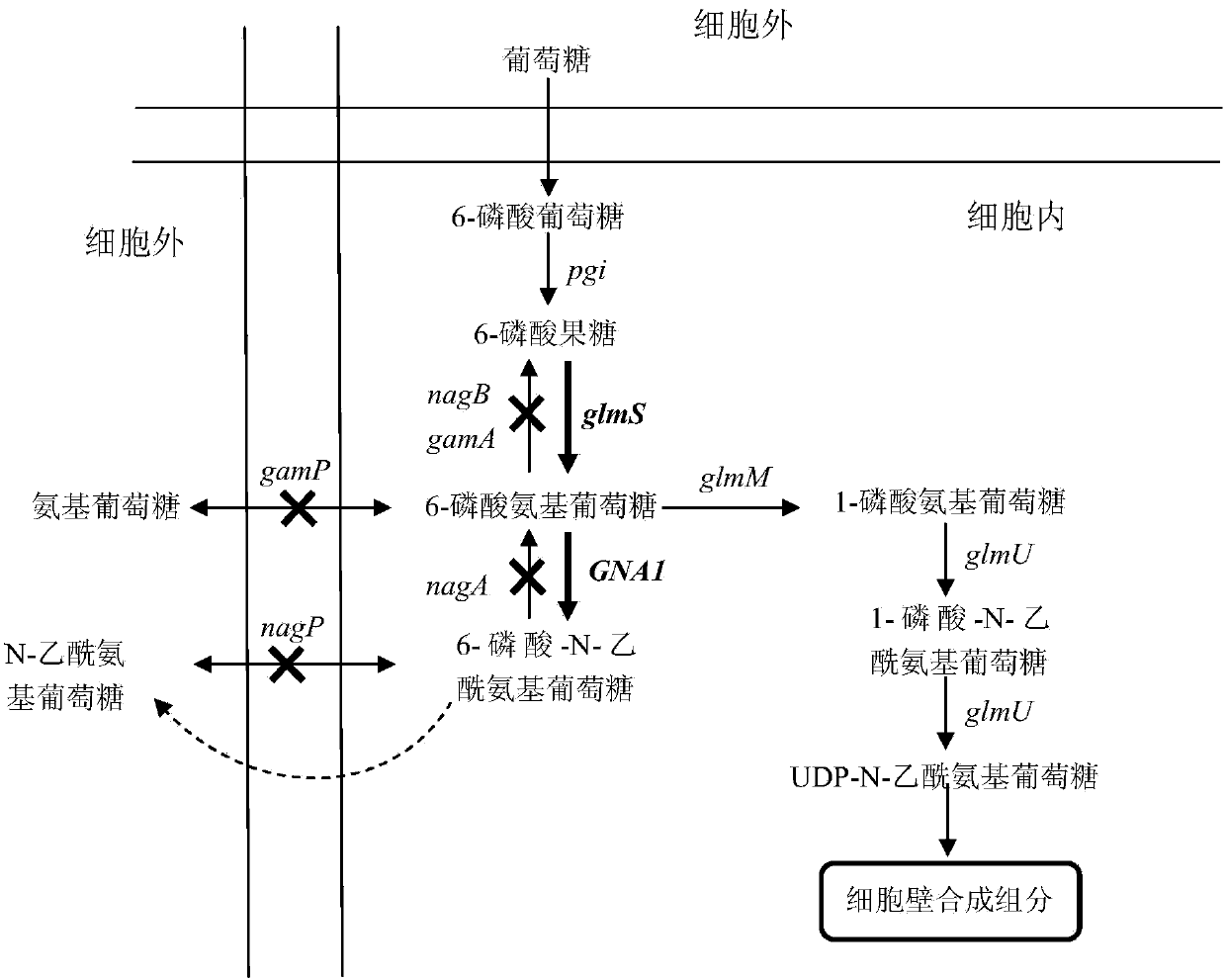
Bacillus subtilis for producing N-acetylglucosamine as well as construction method and application of bacillus subtilis
A technology of Bacillus subtilis and acetamido, which is applied in the field of Bacillus subtilis and its construction, can solve the problems of low yield, unfavorable industrial production application, and high production cost, and achieve enhanced gene expression, high industrial utilization value, and prevention of backflow and the effect of consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Knockout of the nagAB gene cluster in Bacillus subtilis 168 strain
[0035]1. According to the genome sequence of Bacillus subtilis 168 strain (Genbank No.NC_000964), design primers: upstream primer F-nagAB-up-BamHI:CT GGATCC GACTGCAAGATTTCGGCCTGGG (shown in SEQ ID NO.1) and downstream primer R-nagAB: CATAAGTCAGCATGTTCCTTTCACATAGATGATCCGCCTTTCTGG (shown in SEQ ID NO.2). Using the genomic DNA of Bacillus subtilis 168 strain as a template, the upstream 1000bp fragment of the nagAB gene cluster was amplified by PCR.
[0036] 2. According to the Bacillus subtilis 168 strain genome (Genbank No.NC_000964) sequence, design primers: upstream primer F-nagAB:CCAGAAAGGCGGATCATCTATGTGAAAGGAACATGCTGACTTATG (shown in SEQ ID NO.3) and downstream primer R-nagAB-down-NcoI:GCT CCATGG TAACGTATATACCAATGAAGAG (shown in SEQ ID NO. 4). Using the genomic DNA of Bacillus subtilis 168 strain as a template, a 1000 bp fragment downstream of the nagAB gene cluster was amplified by PCR.
[0...
Embodiment 2
[0044] 2. Continue to knock out the gamAP gene cluster in Bacillus subtilis
[0045] 1. According to the genome sequence of Bacillus subtilis 168 strain (Genbank No.NC_000964), design primers: upstream primer F-gamAP-up-BamHI:CT GGATCC ACTGCTCCCCACAGCACTTTTCC (shown in SEQ ID NO.5) and downstream primer R-gamAP: CGCAGCAGGGGGGACTTTTTTACATGTGACACCCCCTCAAAGAG (shown in SEQ ID NO.6). Using the genomic DNA of Bacillus subtilis 168 strain as a template, the upstream 1000bp fragment of the gamAP gene cluster was amplified by PCR.
[0046] 2. According to the sequence of Bacillus subtilis 168 strain genome (Genbank No.NC_000964), design primers: upstream primer F-gamAP:CTCTTTGAGGGGGTGTCACATGTAAAAAAGTCCCCCCTGCTGCG (shown in SEQ ID NO.7) and downstream primer R-gamAP-down-NcoI:GCT CCATGG ATACCACTCGTTTGGGACAGCC (shown in SEQ ID NO. 8). Using the genomic DNA of Bacillus subtilis 168 strain as a template, a 1000 bp fragment downstream of the gamAP gene cluster was amplified by PCR.
...
Embodiment 3
[0051] 3. Continue to knock out the nagP gene in Bacillus subtilis
[0052] 1. According to the genome sequence of Bacillus subtilis 168 strain (Genbank No.NC_000964), design primers: upstream primer F-nagP-up-BamHI:CT GGATCC CAAGACCTCCTCGTACAGAATAATG (shown in SEQ ID NO.9) and downstream primer R-nagP:GGTTGCCCCTCTCCGCTTTTTTACATACCCATCCCCCTCATACCC (shown in SEQ ID NO.10). Using the genomic DNA of Bacillus subtilis 168 strain as a template, the upstream 1000bp fragment of the nagP gene was amplified by PCR.
[0053] 2. According to the sequence of Bacillus subtilis 168 strain genome (Genbank No.NC_000964), design primers: upstream primer F-nagP:GGGTATGAGGGGGATGGGTATGTAAAAAAGCGGAGAGGGCAACC (shown in SEQ ID NO.11) and downstream primer R-nagP-down-NcoI:GCT CCATGG TTCCGGCGATTCTGAAGTCTAAG (shown in SEQ ID NO. 12). Using the genomic DNA of Bacillus subtilis 168 strain as a template, a 1000 bp fragment downstream of the nagP gene was amplified by PCR.
[0054] 3. Take 5ul of eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com