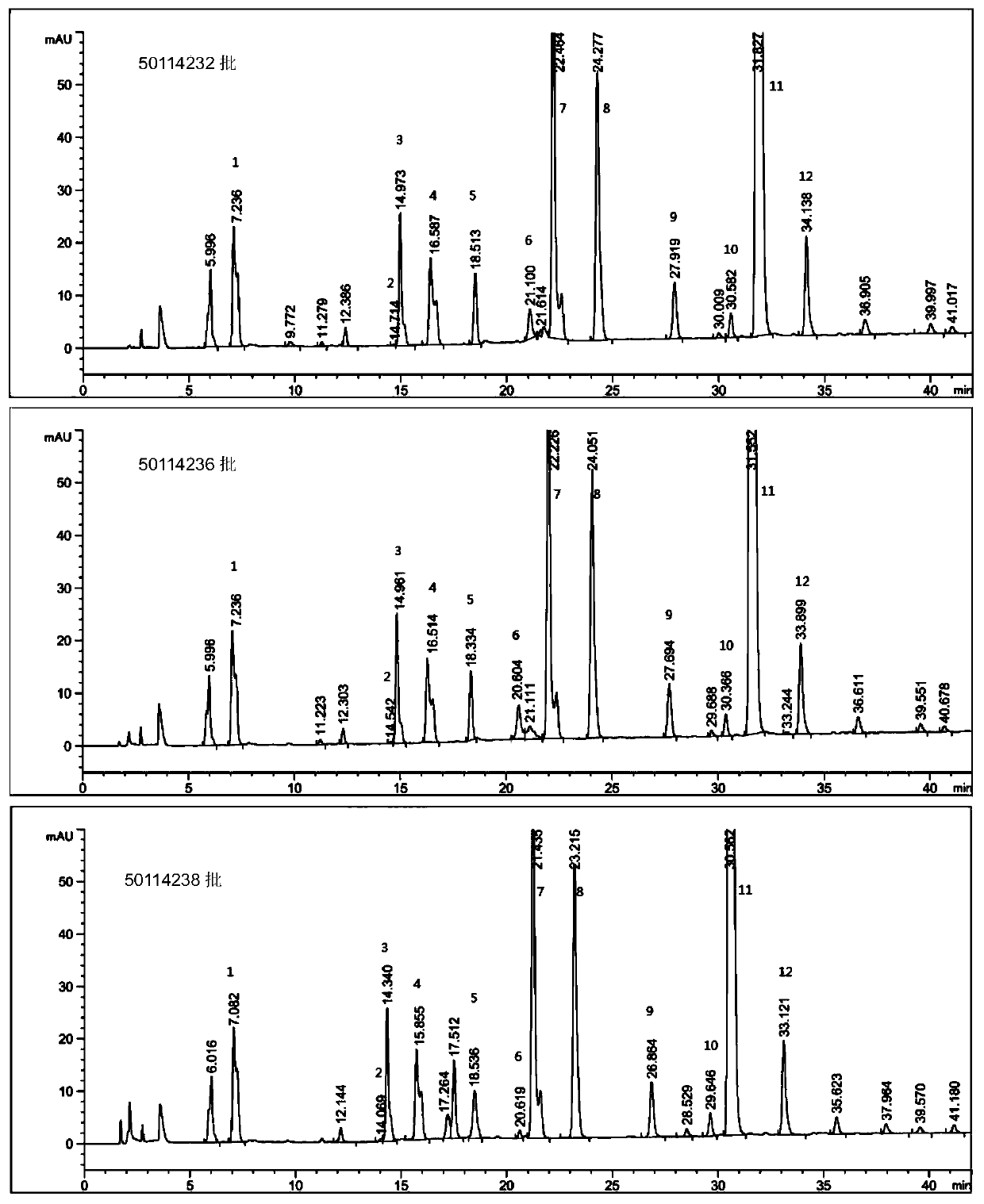
Production method of enoxaparin sodium
A technology of enoxaparin sodium and production method, applied in the production field of enoxaparin sodium, can solve the problems of non-standard structure, unfavorable production and economy, waste of time, manpower and material resources and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Preparation of heparin sodium solution: Accurately weigh 20mg of heparin sodium, put it in a 2mL centrifuge tube, add 1mL of ultrapure water, vortex to dissolve, and shake well to obtain a 20mg / mL heparin sodium solution;
[0032] 2) Preparation of phosphate buffer solution: Accurately weigh 68 mg of potassium dihydrogen phosphate, add 10 mg of bovine serum albumin, put it in a 50 mL volumetric flask, add 30 mL of ultrapure water to dissolve, measure pH, and adjust pH7 with 1M potassium hydroxide if necessary .0, dilute to the mark with ultrapure water, and shake well. Use a syringe to draw a certain volume before each use, and use it after filtering with a 0.45μm filter membrane;
[0033] 3) Mixture of heparanase I, II, and III: Calculate the volume required for each according to the labeled amounts of the three heparinases, absorb the volume and dissolve it in a certain amount of phosphate buffer solution to prepare a heparinase-containing solution. I, II, III con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com