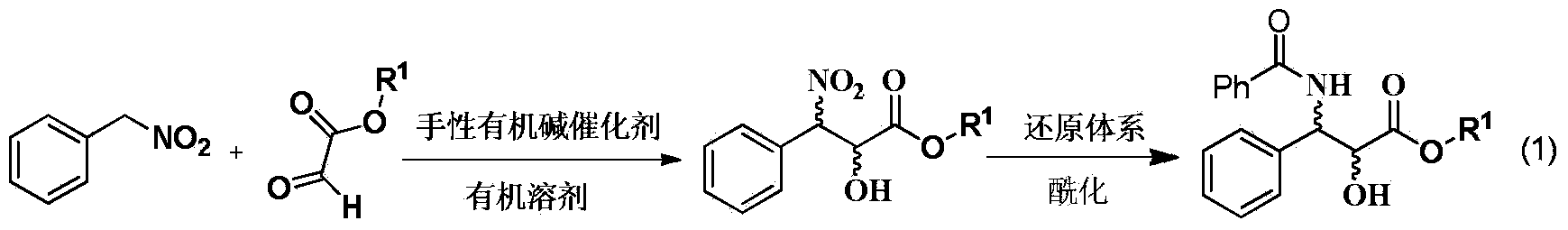
Method for synthesizing chiral 3-amino-3-phenyl-2-hydroxy carboxylate compound
A technology for hydroxycarboxylic acid esters and compounds is applied in the field of synthesizing chiral 3-amino-3-phenyl-2-hydroxycarboxylic acid ester compounds, and can solve the problems of low final yield, long synthesis steps, difficult preparation and the like, Achieve the effect of difficult preparation and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
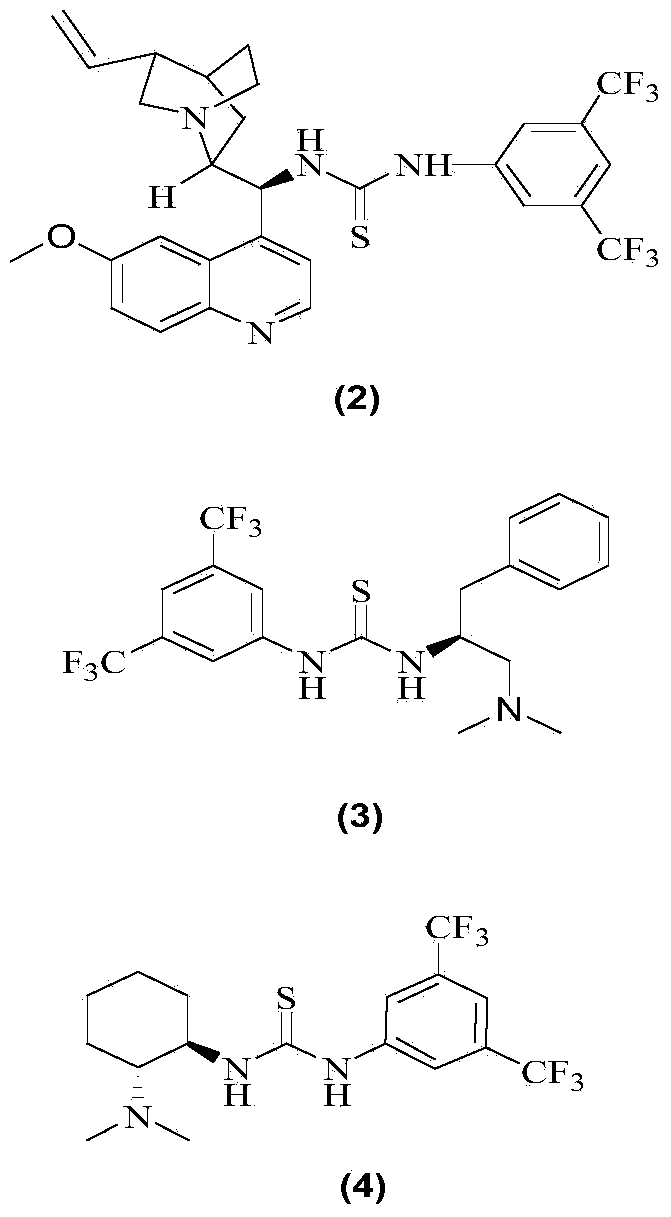
[0018] Add 0.786g (5.72mmol) of nitrobenzyl, 5mL of tetrahydrofuran and 1.24mL (6.3mmol) of ethyl glyoxylate 50% toluene solution into a 25mL round bottom flask, add a magnet, and add 340mg (0.57 mmol) 1-(3,5-ditrifluoromethylphenyl)-3-[(6-methoxy-quinoline-4)-(5-vinyl-1-aza-bridged ring[2.2. 2] Decane-2)-methyl]-thiourea, reacted at room temperature for 18h, evaporated the solvent, and column chromatography (petroleum ether: ethyl acetate 10-5:1) gave a colorless viscous oil as chiral 3 - 1.163 g of ethyl nitro-3-phenyl-2-hydroxycarboxylate, yield 85.1%.
[0019] 1 H NMR (CDCl 3 , 500MHz): δ1.12(t, J=7.5HZ, 3H), 3.18(br, 1H), 4.13-4.23(m, 2H), 4.84(t, J=5.5HZ, 1H), 5.73(d, J=5.5HZ,1H),7.25-7.45(m,3H),7.51-7.53(m,2H).27%ee.
[0020] The result of hydrogen spectrum confirmed that the compound synthesized by the above method was chiral ethyl 3-nitro-3-phenyl-2-hydroxycarboxylate.
Embodiment 2
[0022] Add 0.786g (5.72mmol) of nitrobenzyl, 5mL of tetrahydrofuran and 1.24mL (6.3mmol) of ethyl glyoxylate 50% toluene solution into a 25mL round bottom flask, add a magnet, and add 256mg (0.57 mmol) 1-(1-phenyl-2-dimethylamino-ethyl)-3-(3,5-bistrifluoromethylphenyl)-thiourea, reacted at room temperature for 18h, evaporated the solvent, column layer Analysis (petroleum ether: ethyl acetate 10 ~ 5: 1) to obtain a colorless viscous oil is 3-nitro-3-phenyl-2-hydroxy ethyl carboxylate 1.028g, yield 75.2%; 19%ee .
Embodiment 3
[0024] Add 0.786g (5.72mmol) nitrobenzyl, 5mL tetrahydrofuran and 1.24mL (6.3mmol) ethyl glyoxylate 50% toluene solution into a 25mL round bottom flask, add a magnet, and add 235.4mg ( 0.57mmol) 1-cyclohexyl-3-(2-dimethylamino-1-phenyl-ethyl)-thiourea, 1-(3,5-ditrifluoromethylphenyl)-3-(2- Dimethylamino-cyclohexyl)-thiourea, reacted at room temperature for 18h, evaporated the solvent, and column chromatography (petroleum ether: ethyl acetate 10-5:1) gave a colorless viscous oil as 3-nitro-3- Ethyl phenyl-2-hydroxycarboxylate 1.028g, yield 77.2%; 11% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com