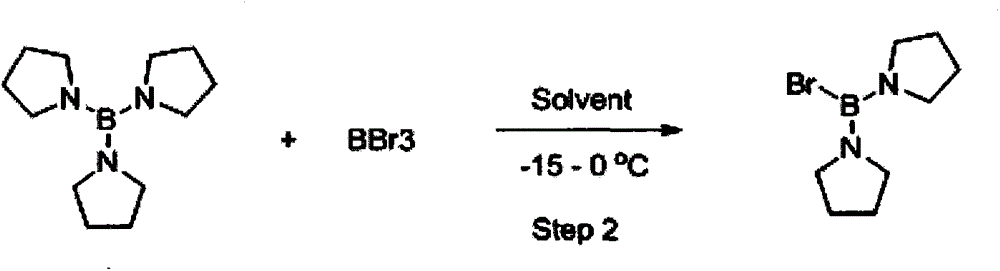Method for synthesizing bisdiboron
A technology of biboronic acid pinacol ester and pinacol, which is applied in the field of boron chemical synthesis, can solve problems such as inappropriateness, and achieve the effects of easy operation and reduced environmental hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
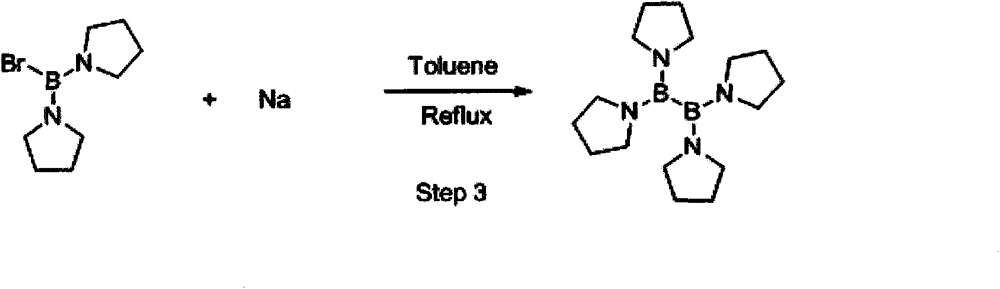
[0038] Synthesis of tri(tetrahydropyrrole) boron (1):
[0039] After argon replacement, add 110 kg of anhydrous toluene to the 500L enamel reaction kettle, cool the system down to -15°C, add triethylamine (80.9 kg, 800 moles) and tetrahydropyrrole (55.0 kg, 775 moles) , after stirring evenly, wait for dropwise addition. After argon replacement, add 20 kg of anhydrous toluene to another 200L enamel kettle, cool the system down to -15°C, add 62.6 kg of boron tribromide (250 moles), stir well and keep for 20-30 minutes. Under the protection of the argon system, slowly add the boron tribromide toluene solution into a 500L enamel reaction kettle. During the process of adding boron tribromide, the system begins to produce salt, and then gradually produces a large amount of solids.
[0040] The entire addition process takes about 6-8 hours, the temperature control should not exceed 0°C, and the stirring should be stable and uniform. After the addition is complete, keep stirring at ...
Embodiment 2
[0043] Synthesis of tri(tetrahydropyrrole) boron (1):
[0044] After nitrogen replacement, 130 kg of n-heptane was added to a 500L stainless steel reactor, the system was cooled to -15°C, triethylamine (83.5 kg, 825 moles) and tetrahydropyrrole (58.5 kg, 825 moles) were added, After stirring evenly, wait for dropwise addition. After nitrogen replacement, add 40 kg of n-heptane to another 200L stainless steel, cool the system down to -15°C, add 62.6 kg of boron tribromide (250 moles), stir well and keep for 20-30 minutes. Under the protection of the argon system, slowly add the boron tribromide toluene solution into a 500L enamel reaction kettle. During the process of adding boron tribromide, the system begins to produce salt, and then gradually produces a large amount of solids.
[0045] The entire addition process takes about 8-12 hours, the temperature control should not exceed 0°C, and the stirring should be stable and uniform. After the addition is complete, keep stirrin...
Embodiment 3
[0048] Synthesis of di(tetrahydropyrrole) boron bromide (2):
[0049] After nitrogen replacement, in the 500L enamel reaction kettle, add the toluene solution containing compound 1 (48.1 kg, 217 moles) in the first step, cool the system to -10 ° C, and add 27.1 kg of boron tribromide (108.5 moles, 0.5 equivalents) ) was added, stirred evenly and maintained for 20-30 minutes. The whole addition process takes about 3-5 hours, and the temperature control does not exceed 0°C. After the addition is complete, keep stirring at -10 to 0°C for 3 hours.
[0050] After the reaction, about 225 kilograms of the reaction solution was obtained, and the reaction yield of this step was 91%, which contained 68.4 kilograms of pure di(tetrahydropyrrole) boron bromide (2), which could directly enter the next step reaction. Analysis method: take the reaction solution and titrate it with silver nitrate solution. The pure product can take 200~500 milliliters of reaction liquids, the toluene rotary...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap