Synthesis and separation method of trans-seven-membered-cucurbituril
A seven-membered melon ring, separation method technology, applied in the direction of organic chemistry, etc., to achieve the effect of simple and fast method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
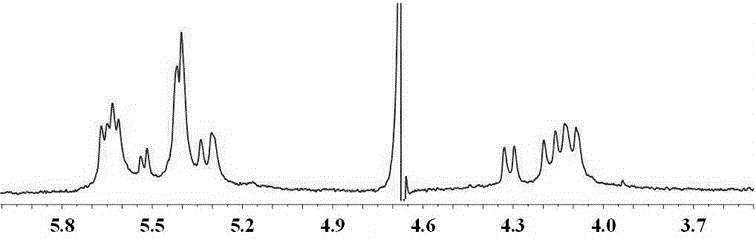
[0019] Embodiment: The synthetic implementation method of trans seven-membered melon ring iQ[7]:
[0020] Weigh 50 grams of glycoside urea, 20 grams of paraformaldehyde, 200 mL of concentrated hydrochloric acid, reflux for 6 hours, cool, and dilute with water step by step until no precipitation occurs (the precipitation is eight-membered melon ring Q[8], six Yuan melon ring Q[6] and other poorly soluble melon rings, about 40 grams). Concentrate the mother liquor containing five-membered melon ring Q[5], seven-membered melon ring Q[7], a small amount of spliced fourteen-membered melon ring tQ[14], and trans seven-membered melon ring iQ[7] to a certain volume ( It should not be too viscous, so as not to affect the sample on the column, take 25 mL of the concentrated solution (0.9-1.1 grams of solid per mL). Load the concentrated solution on a chromatography column with silica gel G as the stationary phase (diameter: 40mm; height: 800mm) , with 1:1:0.01~0.3 water: acetic acid:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com