Bis(dithio hydrocarbon)-2,5-bis(1,3-dithiol-2-ylidene)-1,3,4,6-tetrathiapentalene (TTP) organic conductive crystal and preparation method thereof
A technology of fullvalene type and bis-disulfide, applied in the field of bis-disulfide-double-fused tetrathiafulvalene type organic conductive crystal and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
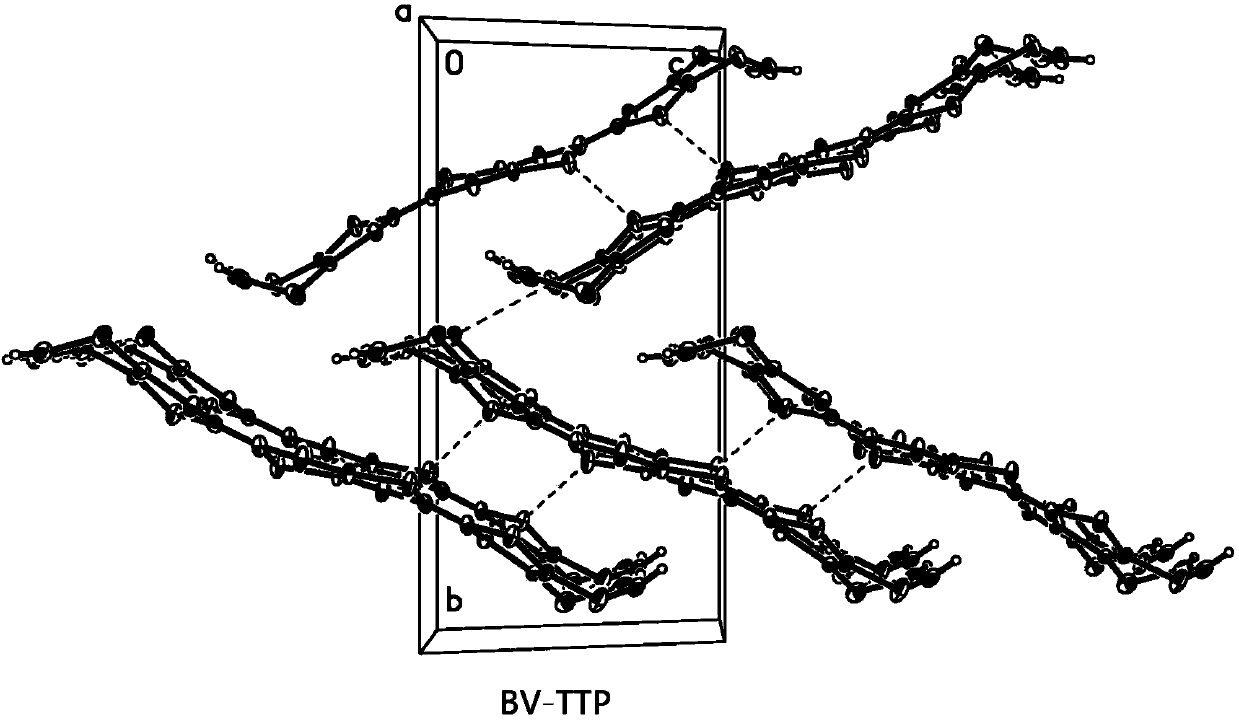
[0071] Embodiment 1, double disulfide vinylidene-double fused tetrathiafulvalene (BV-TTP)
[0072] The synthetic route shows:
[0073]
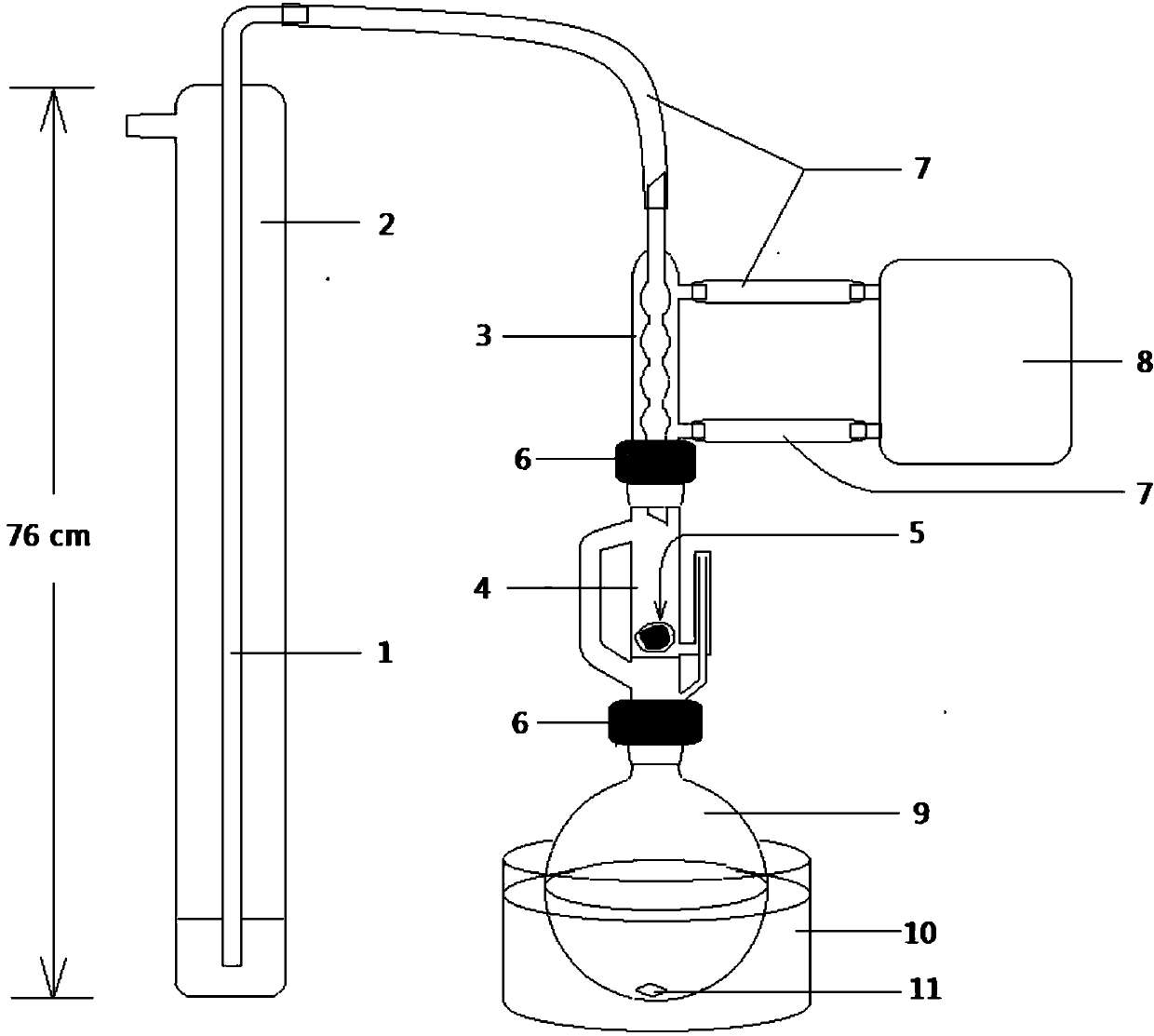
[0074] 0.089g (0.40mmol) of the small precursor compound was placed in a 50mL three-necked round-bottomed flask, and the nitrogen gas was pumped and changed 3 times. Under the protection of dry nitrogen, add 10mL of freshly steamed toluene, heat and stir until completely dissolved, and naturally cool to 50°C; add 0.101g (0.26mmol) of the large precursor compound to the above solution, and immediately add 7mL of freshly steamed coupling agent with a syringe P(OMe) 3 , pump and change nitrogen 3 times; accelerate stirring, heat up to 108°C, start to boil, reflux under nitrogen for 20 hours, cool naturally, then put in refrigerator -10°C overnight, then add 5mL of methanol, filter and dry to get reddish brown Crude solid 0.094 g. Filtrate dot plate (carbon disulfide as developing agent) found: there is a small precursor compound, indicatin...
Embodiment 2
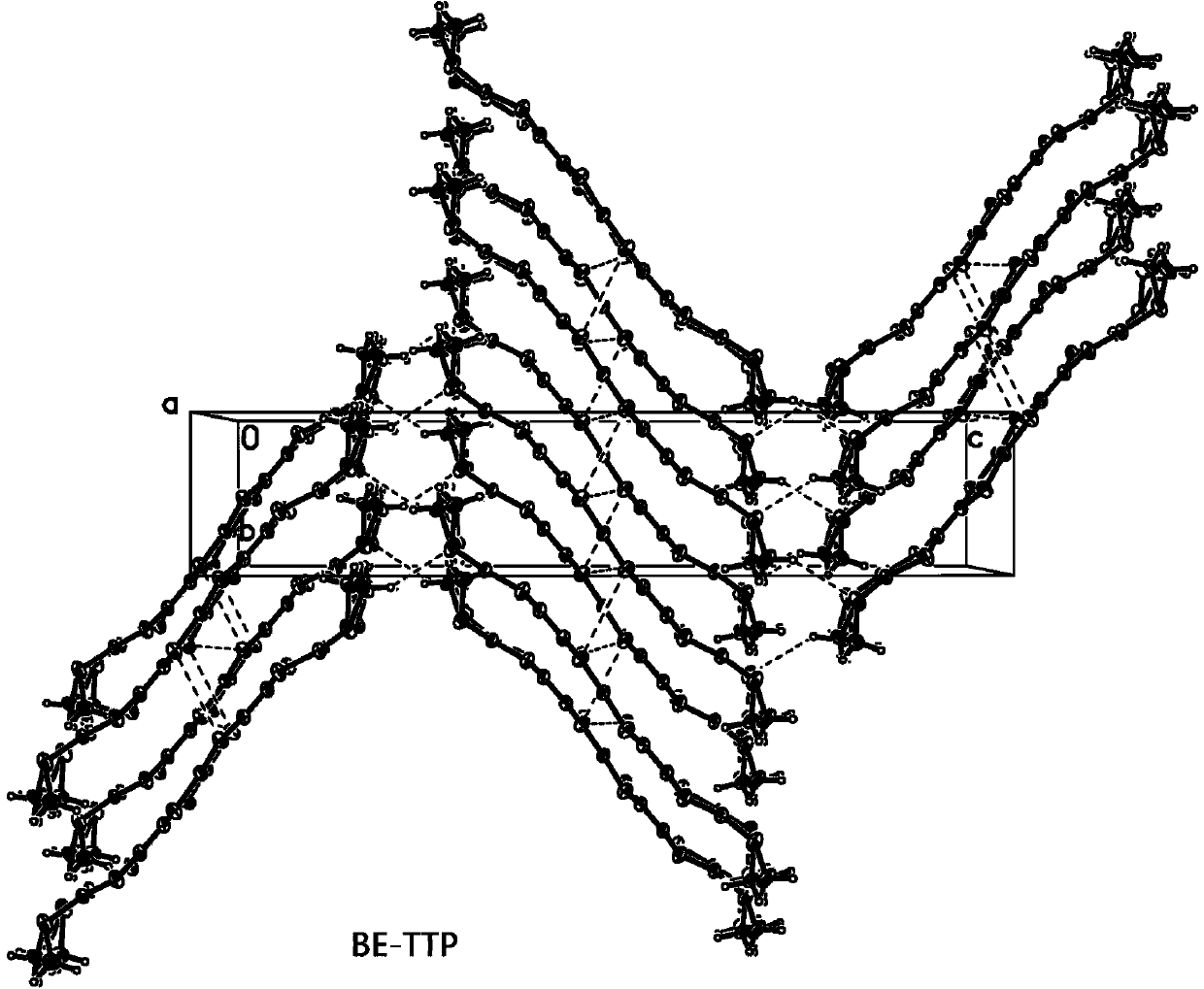
[0088] Embodiment 2, double disulfide ethylene-double fused tetrathiafulvalene (BE-TTP)
[0089] The synthetic route shows:
[0090]
[0091] Preparation method is as embodiment 1, and difference is:
[0092] The reactants were: 0.088g (0.39mmol) of the small precursor compound, 0.103g (0.27mmol) of the large precursor compound, the reflux time was 22 hours under nitrogen, and 0.077g of a reddish-brown solid was obtained after suction filtration and drying. The filtrate was plated, and no small precursor compounds were found.
[0093] The reddish-brown solid was placed in a Soxhlet extractor for the first 5-day extraction with carbon disulfide. The extract is separated by column chromatography. First, the redistilled dichloromethane is used as the eluent to elute the self-conjugate BE-TTF of the small precursor compound (see the background technology part for the structural formula); then carbon disulfide is used as the eluent , the obtained eluate was slowly concentrate...
Embodiment 3
[0102] Example 3, disulfide ethylene-disulfide methylene-double fused tetrathiafulvalene (EM-TTP)
[0103] The synthetic route shows:
[0104]
[0105] Preparation method is as embodiment 1, and difference is:
[0106] The reactants were: 0.087g (0.41mmol) of the small precursor compound and 0.104g (0.27mmol) of the large precursor compound. After 18 hours of reaction, they were placed in the refrigerator overnight to obtain 0.049g of a reddish-brown solid. The filtrate was plated, and no small precursor compounds were found.
[0107]The above solid was placed in a Soxhlet extractor, and the first extraction was carried out with carbon disulfide for a period of 5 days. The extract was separated by column chromatography, and the self-conjugate BM-TTF (structural formula is as follows) of the small precursor was eluted with redistilled dichloromethane as the eluent; then carbon disulfide was used as the eluent to elute the corresponding After dehydration and slow concentra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inner diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com