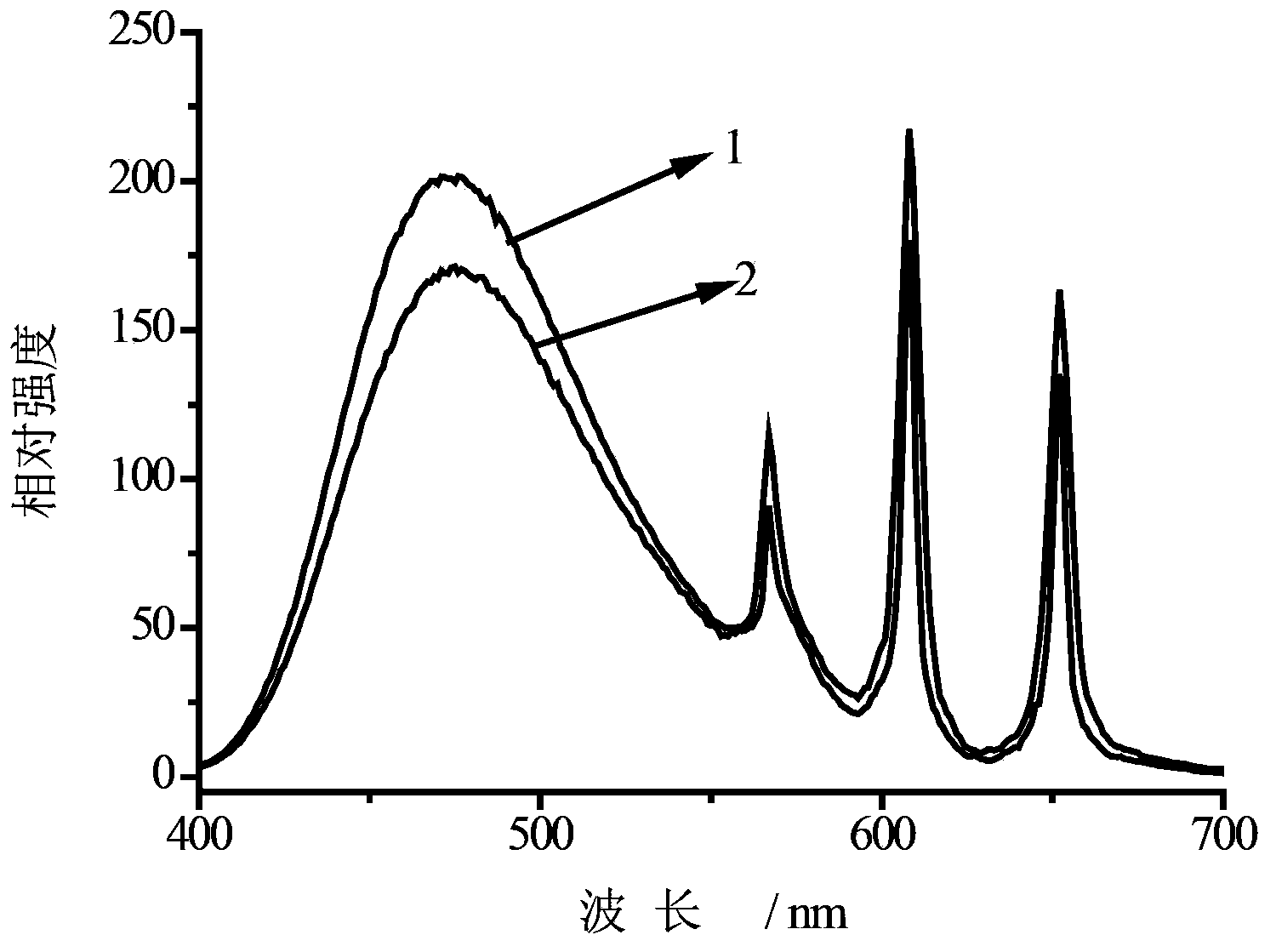
Samarium-doped hollow-structure Sr2CeO4 luminescent material and preparation method thereof
A technology of luminescent materials and hollow structures, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of low luminous intensity of phosphor powder, inconsistent powder particle size, uneven particle distribution, etc., achieving low cost and not harsh process conditions , the effect of fewer process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of Hollow Spherical Sr by Precipitation Method 1.995 CeO 4 :Sm 0.005 3+ :
[0029] Weigh 4g of glucose and dissolve it in absolute ethanol to make the solution volume 40mL, then transfer the solution into a 50mL reaction kettle with a polytetrafluoroethylene liner, cover and tighten it, and react at 120°C for 36h to prepare carbon The bead solution was washed several times with deionized water and absolute ethanol, centrifuged, and dried at 60°C to obtain carbon bead C coated with metal nanoparticles for use;
[0030] Take by weighing 10.3630g SrO and dissolve in nitric acid to obtain 100mL1mol / L of Sr(NO 3 ) 2 Solution; Weigh 17.2120g CeO 2 Dissolved in nitric acid to obtain 100mL1mol / L Ce(NO 3 ) 3 solution; weigh 0.0872g Sm 2 o 3 Dissolved in nitric acid to obtain 100mL0.005mol / L of Sm(NO 3 ) 3 solution.
[0031] According to Sr 1.995 CeO 4 :Sm 0.005 3+ stoichiometric ratio, pipette 7.98mL1mol / L Y(NO 3 ) 3 , 4mL1mol / L Ce(NO 3 ) 3 and 4mL...
Embodiment 2
[0034] Preparation of Hollow Spherical Sr by Precipitation Method 1.9 CeO 4 :Sm 0.1 3+
[0035] Weigh 0.003g of glucose and dissolve it in absolute ethanol to obtain 40mL of glucitol solution, transfer this solution into a 50mL reaction kettle with a polytetrafluoroethylene liner, cover and tighten it, and react at 150°C for 10h to prepare carbon The bead solution was centrifuged to obtain a solid phase, washed twice with deionized water and absolute ethanol, and dried at 70°C to obtain carbon bead C.
[0036] According to Sr 1.9 CeO 4 :Sm 0.1 3+ stoichiometric ratio, pipette 15.2mL0.5mol / L Sr(CH 3 COO) 2 solution, 8mL0.5mol / L Ce(CH 3 COO) 3 solution and 0.2mL2mol / L Sm(CH 3 COO) 3 Solution, placed in a 100mL beaker to form a mixed solution, then weighed carbon pellets C0.6mg into the mixed solution, and stirred evenly. Under magnetic stirring, 10mL precipitant oxalic acid solution (2mol / L) was added dropwise, and the pH was adjusted to 10 by ammonia water. After ...
Embodiment 3
[0039] Preparation of Hollow Spherical Sr by Precipitation Method 1.99 CeO 4 :Sm 0.01 3+
[0040] Weigh 5g of glucose and dissolve it in absolute ethanol to obtain 40mL of glucitol solution, transfer this solution into a 50mL reaction kettle with a polytetrafluoroethylene liner, cover it and tighten it, and react at 180°C for 24h to prepare carbon small The solution of the spheres was centrifuged to obtain a solid phase, washed twice with deionized water and absolute ethanol, and dried at 60°C to obtain carbon spheres C.
[0041] According to Sr 1.99 CeO 4 :Sm 0.01 3+ stoichiometric ratio, pipette 7.96mL1mol / L Sr(NO 3 ) 2 solution, 8mL0.5mol / L Ce(NO 3 ) 3 solution and 4mL0.01mol / L Sm(NO 3 ) 3 solution, placed in a 100mL beaker to form a nitric acid mixture, then weighed 120mg of carbon pellets C and added to the mixture, and stirred evenly. Under magnetic stirring, dropwise add 40mL precipitant oxalic acid solution (0.5mol / L), then adjust the pH to 9 with ammonia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com