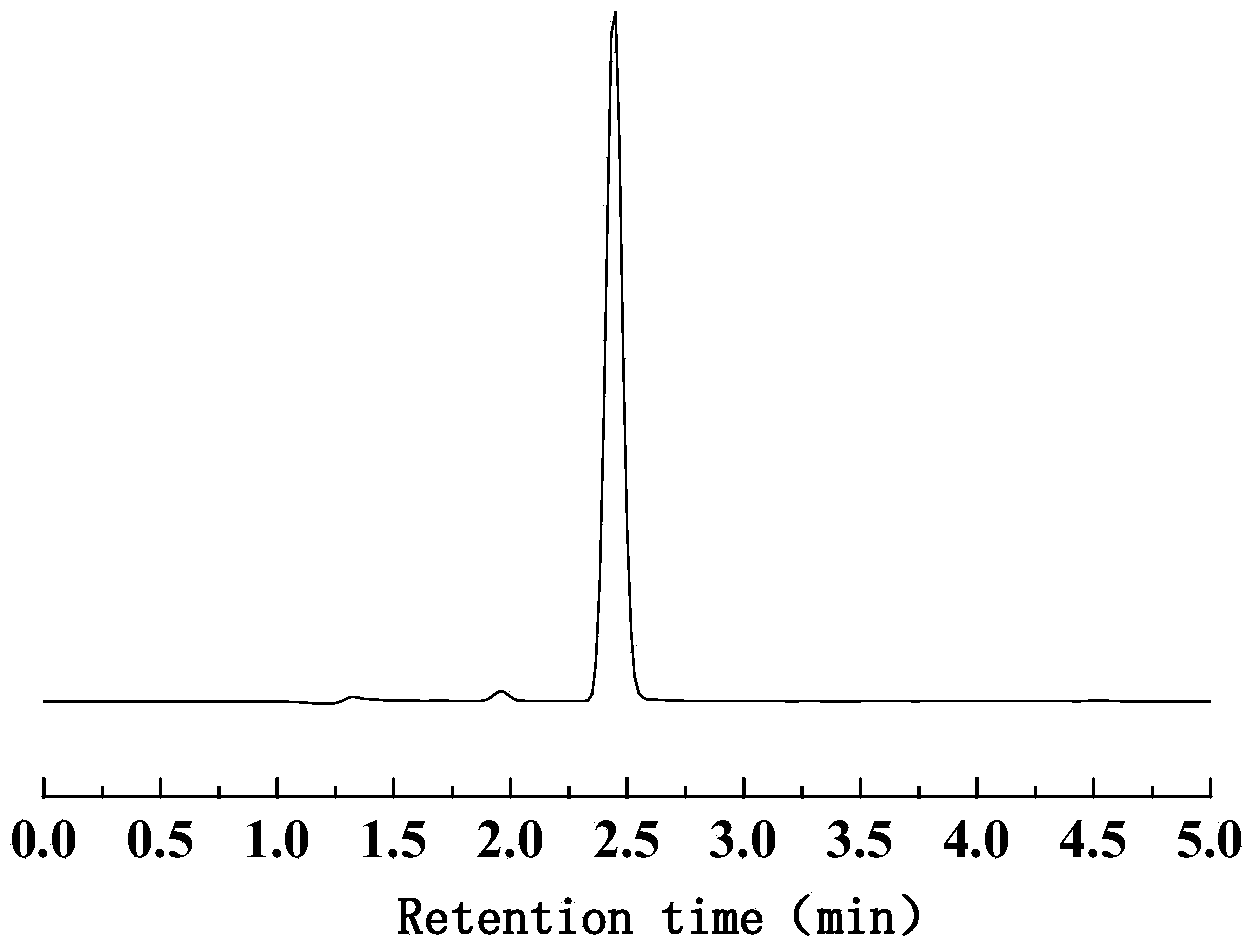
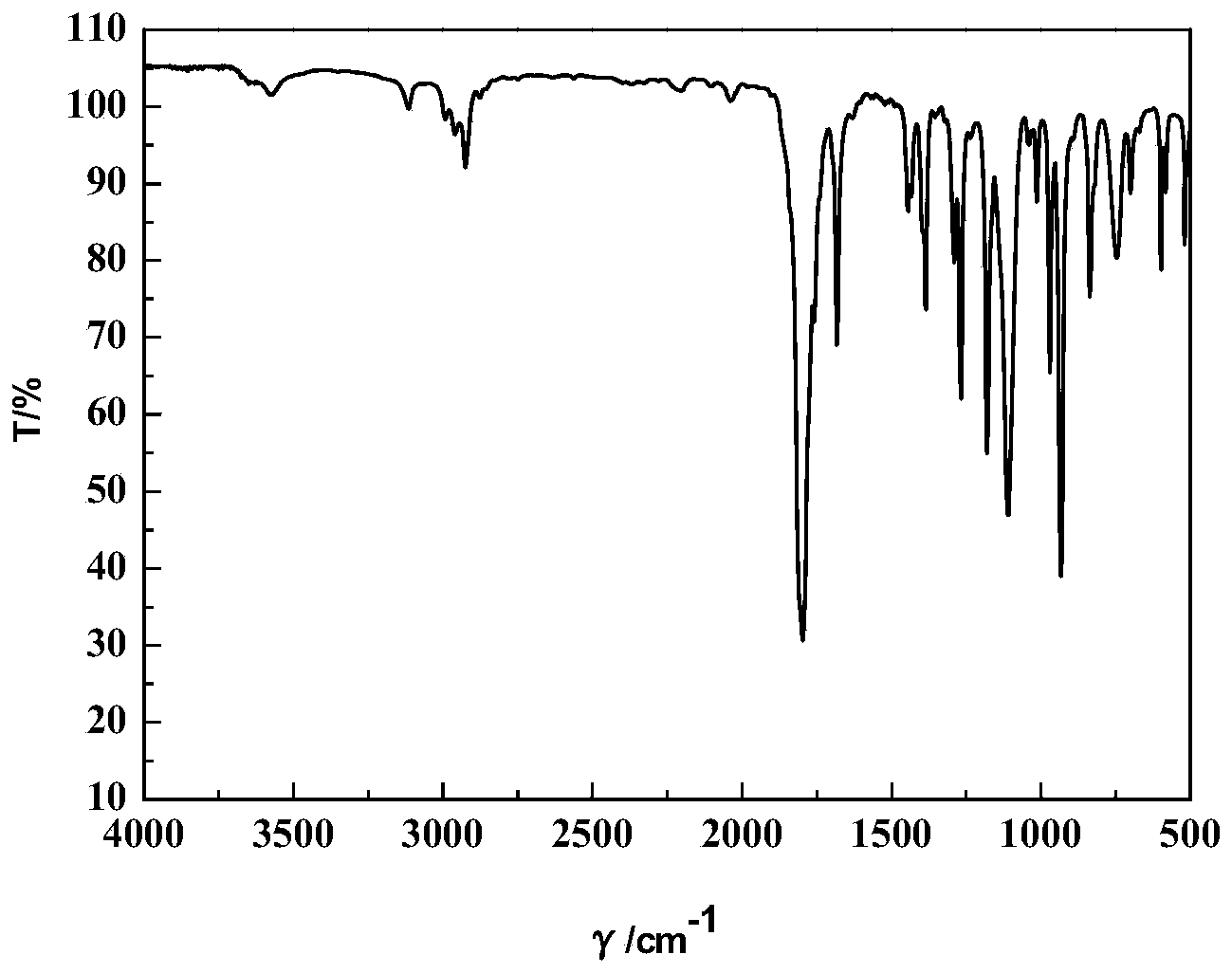
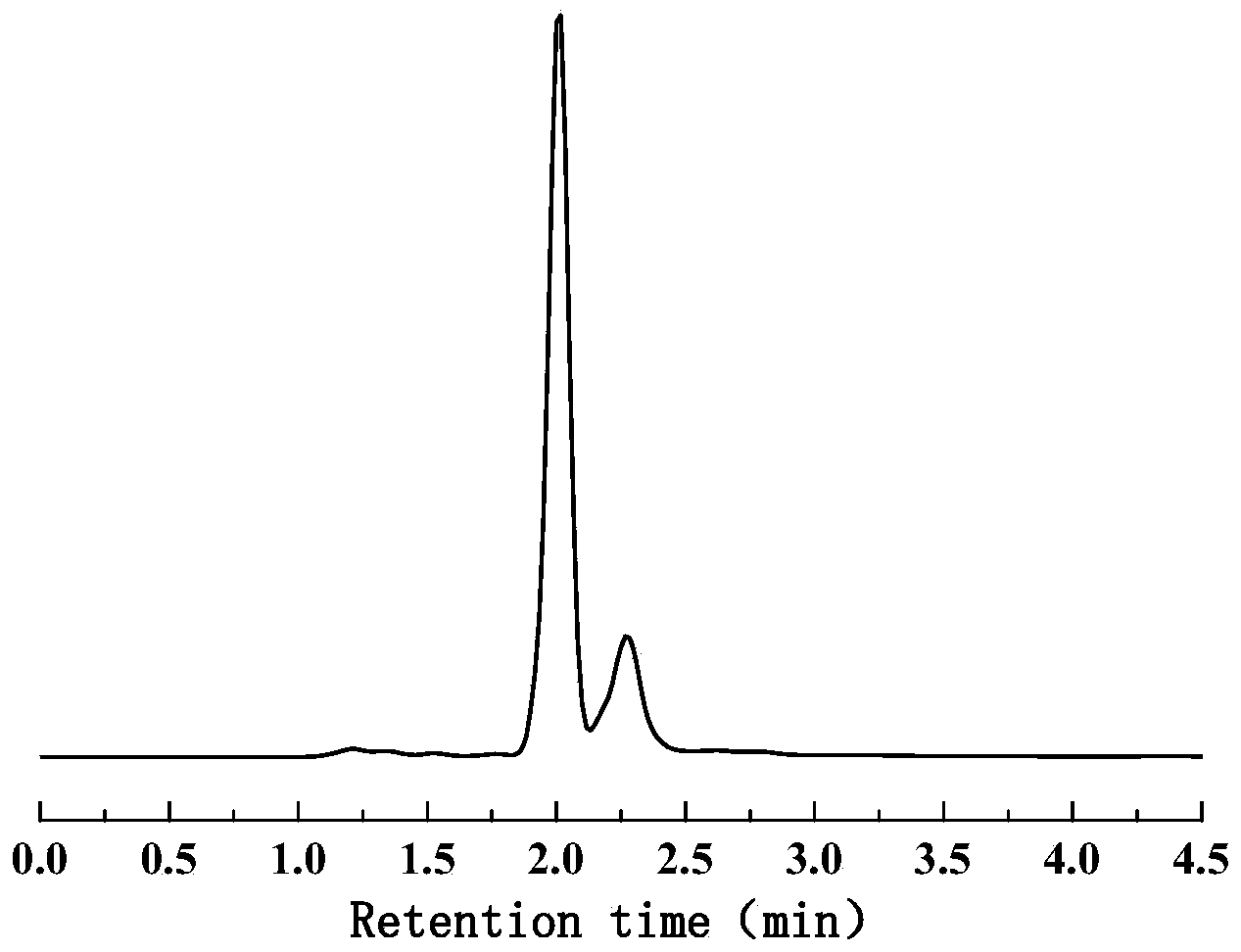
Method for preparing biological gasoline from biomass
A bio-gasoline, biomass technology, used in fuels, petroleum industry, liquid carbonaceous fuels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0028] The reaction equation for preparing levulinic acid by hydrolysis of biomass is as follows:
[0029] (C 6 H 10 O 5 ) n →nC 5 H 8 O 3 (Levulinic acid)+nCH 2 O 2
[0030] Put the raw material biomass (sawdust, bagasse, sawdust, cellulose, straw, absorbent cotton, fructose, glucose or starch) and 5% hydrochloric acid aqueous solution with a mass concentration of 5% into the autoclave at a mass ratio of 1:10. At 140°C, 200°C or 220°C, the hydrolysis reaction is carried out for 5, 10 or 240 minutes, and the residue is filtered off after cooling to obtain a biomass hydrolysate. The product is qualitatively and quantitatively analyzed by waters UPLC. The reaction conditions and results of biomass hydrolysis to prepare levulinic acid are shown in Table 1, No. 1-8.
[0031] Table 1 Reaction conditions and results for the preparation of levulinic acid by hydrolysis of biomass in Examples 1-8
[0032] Example
[0033] 2. Preparation of Angelica Lactone by Dehydration of Levulinic Acid...
Embodiment 9-13
[0035] The reaction equation of levulinic acid dehydration to prepare angelica lactone is as follows:
[0036] C 5 H 8 O 3 (Levulinic acid)→C 5 H 6 O 2 (Angelica lactone)+H 2 O
[0037] Weigh 25g of levulinic acid into the flask, and add 3% of its mass of catalyst H 3 PO 4 Or ZSM-5, heat up to 130°C, 160°C, 175°C or 180°C under stirring conditions, react for 60min, 120min, 150min or 180min, control the vacuum degree of the reaction system, and return the levulinic acid in the gas phase of the reaction system to the reaction In the system, angelica lactone vapor is distilled from the distillation head, and the condensed liquid is collected. The product uses watersUPLC for qualitative and quantitative analysis. The reaction conditions and results of the dehydration of levulinic acid to prepare angelica lactone are shown in Table 2 with No. 9-13.
[0038] Table 2 Example 9-13 Reaction conditions and results of levulinic acid dehydration to prepare angelica lactone
[0039] Example ...
Embodiment 14-21
[0042] The reaction equation for preparing angelica lactone polymer by polymerization of angelica lactone is as follows:
[0043] nC 5 H 6 O 2 (Angelica lactone)→C 5n H 6n O 2n (Angelica lactone polymer)
[0044] Weigh 30g of angelica lactone into the flask, raise the temperature to 60°C, 80°C, 100°C, 120°C, 140°C or 160°C, add 3% by mass of catalyst K under stirring conditions 2 CO 3 , React for 1min, 5min, 30min, 120min or 180min to obtain angelica lactone polymer. The product is qualitatively and quantitatively analyzed by waters UPLC. The reaction conditions and results of angelica lactone polymerization to prepare angelica lactone polymer are shown in Table III No. 14-21.
[0045] Table 3 Example 14-21 Angelica lactone polymerization reaction conditions and results
[0046]
[0047] 4. Hydrogenation of angelica lactone polymer to produce biogasoline
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com