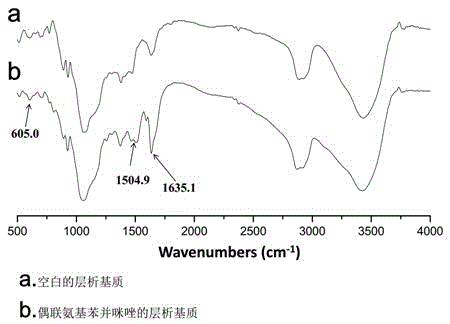
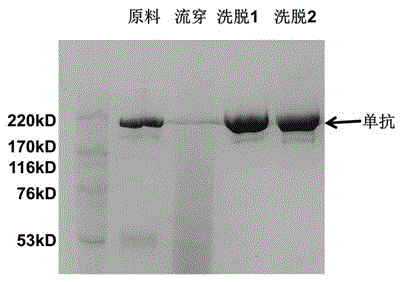
Chromatography medium with aminobenzimidazole as functional ligand and preparation method thereof
An aminobenzimidazole, chromatographic medium technology, applied in chemical instruments and methods, other chemical processes, alkali metal oxides/hydroxides, etc., can solve the problem of low pH, limited electrostatic repulsion, antibody Activity loss and other problems, to achieve the effect of convenient elution, less non-specific adsorption of the medium, and fewer operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take 10 g of drained agarose gel, add 2 g of 20% (v / v) dimethyl sulfoxide, 1 g of allyl bromide and 1 g of sodium hydroxide, activate in a shaker at 150 rpm at 25 °C for 48 hours, filter with suction, and use Wash with deionized water to obtain the activated matrix; then mix the activated matrix and 1g N-bromosuccinimide for bromoalcoholization, react in a 150rpm shaker at 25°C for 5 hours, filter with suction, and wash with deionized water; then bromine The substitute matrix was mixed with 1g of 2-aminobenzimidazole and 1M sodium carbonate buffer (pH12), and reacted in a shaker at 150rpm at 25°C for 24 hours; finally, the medium was suction-filtered, washed with deionized water, added to 10g of ethanolamine, and kept at 25°C React in a shaker at 150 rpm for 8 hours, wash with deionized water, and obtain a chromatographic medium with 2-aminobenzimidazole as a ligand, and the ligand density is 40 μmol / ml.
Embodiment 2
[0028] Take 10 g of drained agarose gel, add 10 g of 20% (v / v) dimethyl sulfoxide, 10 g of allyl bromide, and 5 g of sodium hydroxide, activate it in a shaker at 150 rpm at 25 ° C for 8 hours, filter it with suction, and use it Wash with ionic water to obtain the activated matrix; then mix the activated matrix and 5g N-bromosuccinimide for bromoalcoholization, react in a shaker at 150rpm at 25°C for 1 hour, filter with suction, and wash with deionized water; then bromine The substitute matrix was mixed with 3g of 2-aminobenzimidazole and 0.5M sodium carbonate buffer (pH10), and reacted in a shaker at 150rpm at 25°C for 8 hours; finally, the medium was suction-filtered, washed with deionized water, and added to 50g of ethanolamine, 25 React at 150 rpm shaker at ℃ for 2 hours, wash with deionized water, and obtain a chromatographic medium with 2-aminobenzimidazole as a ligand, and the ligand density is 150 μmol / ml.
Embodiment 3
[0030] Take 10 g of drained agarose gel, add 2 g of 20% (v / v) dimethyl sulfoxide, 1 g of allyl bromide and 1 g of sodium hydroxide, activate in a shaker at 150 rpm at 25 °C for 48 hours, filter with suction, and use Wash with deionized water to obtain the activated matrix; then mix the activated matrix and 1g N-bromosuccinimide for bromoalcoholization, react in a 150rpm shaker at 25°C for 5 hours, filter with suction, and wash with deionized water; then bromine The substitute matrix was mixed with 1g of 4-aminobenzimidazole and 1M sodium carbonate buffer (pH12), and reacted in a shaker at 150rpm at 25°C for 24 hours; finally, the medium was suction-filtered, washed with deionized water, added to 10g of ethanolamine, and kept at 25°C React in a shaker at 150 rpm for 8 hours, wash with deionized water, and obtain a chromatographic medium with 4-aminobenzimidazole as a ligand, and the ligand density is 40 μmol / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com