A kind of preparation method of cefotaxime sodium
A technology of cefotaxime sodium and cefotaxime acid, which is applied in the field of raw material drug preparation in medical technology, can solve the problems of poor fluidity, poor fluidity of cefotaxime sodium raw material drug, etc., and achieve the effect of improving fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
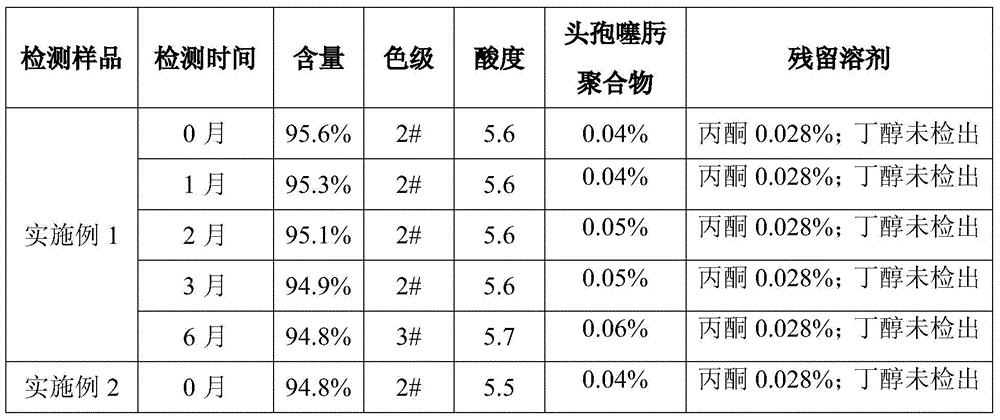
[0029] Control the temperature at 26°C, add 350g of anhydrous sodium acetate to 500mL of purified water, stir to dissolve, add 1000g of cefotaxime acid, stir until the solution is clear, add 2g of activated carbon for decolorization for 10min, then use a sterile membrane to filter, and pour into the filtrate Add 500 mL of butanol, stir for 5 minutes, control the stirring at 150 rpm, control the temperature at 12°C, add acetone, and observe the change of the solution. When solids are found in the solution, stop adding acetone, stop stirring, and add acetone to share 1h, stand still for crystal growth for 30min, then start stirring, control the stirring speed at 150 rpm, control the temperature at 12°C, continue to feed 3L of acetone within 2 hours, after the acetone is added, cool the feed solution to 0°C, and raise After crystallization for 50 minutes, the feed liquid was suction-filtered, the filter cake was washed three times with acetone, placed in an oven, controlled at a t...
Embodiment 2
[0031] Control the temperature at 30°C, add 3.25kg of anhydrous sodium acetate to 4.64L of purified water, stir to dissolve, add 10kg of cefotaxime acid, stir until the solution is clear, add 18g of activated carbon for decolorization for 10min, and then use a sterile membrane to filter, Add 4.64 L of butanol to the filtrate, stir for 5 minutes, control the stirring at 180 rpm, control the temperature at 18°C, add acetone, observe the change of the solution, stop adding acetone, stop stirring, and add When sharing acetone for 1.5 hours, let the crystal grow for 40 minutes, then start stirring, control the stirring speed at 180 rpm, and control the temperature at 18°C. Continue to add 32.48L of acetone within 3 hours. After the acetone is added, cool down the temperature of the feed solution to 5°C, grow the crystal for 60 minutes, filter the feed liquid with suction, wash the filter cake three times with acetone, put it in an oven, control the temperature at 63-65°C, and dry un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com