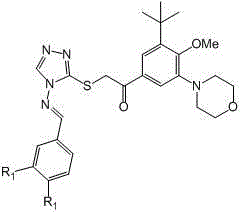
Compounds with terminally-disubstituted triazole Schiff base structure as well as preparation methods and applications of compounds
The technology of a compound and general formula is applied in the fields of triazole Schiff base compounds and their derivatives, PAR-1 antagonists with triazole Schiff base structure and their preparation, which can solve the problems of high bleeding risk and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] .
[0033] Reaction raw materials: self-made, conventional method.
[0034] 1.66 g (10 mmol) compound II and 1.16g (10 mmol) of compound III-1 Dissolve in 20 mL of glacial acetic acid and heat to reflux overnight. After the reaction mixture was slightly cooled, it was poured into 200 mL of ice water, stirred, and the solid was collected by suction filtration, recrystallized from absolute ethanol, and vacuum-dried at room temperature to obtain the product IV-1 , white crystals. MS, m / z = 265 ([M+H] + ).
[0035] 1.33 g (5 mmol) compound IV-1 , 1.85 g (5 mmol) compound V and 2.07 g (15 mmol) of solid potassium carbonate were stirred overnight in 15 mL of acetonitrile, and then heated to reflux for 3 hours. The reaction mixture was cooled slightly and poured into 200 mL of ice water, stirred, adjusted to pH = 4 with concentrated hydrochloric acid, extracted with 50 mL × 3 dichloromethane, combined organic phases, washed with brine, dried over an...
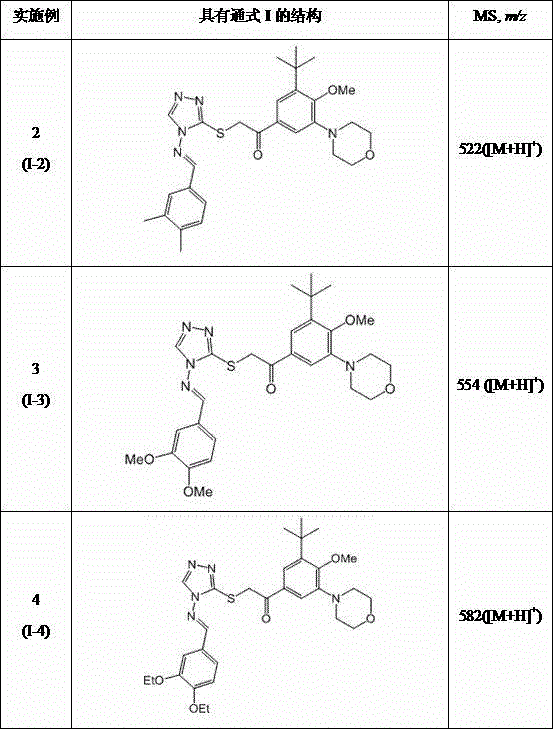
Embodiment 2-4
[0037] Referring to the method of Example 1, it is possible to synthesize I of the following compounds:
[0038]
Embodiment 5
[0039] Example 5 In vitro platelet aggregation inhibition test
[0040] compound Inhibition of Platelet Aggregation IC50 (nM) Compound I-1 1.7 Compound I-2 3.9 Compound I-3 5.1 Compound I-4 3.7
[0041] It can be seen from the above table that the compound of the present invention exhibits obvious inhibitory effect in the platelet aggregation test.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com