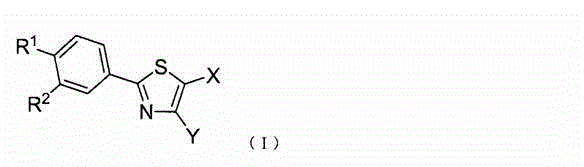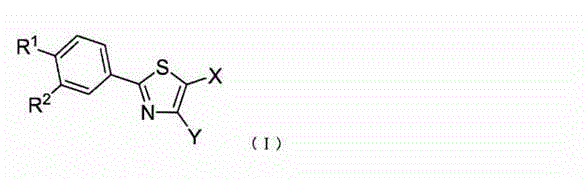Therapeutic agent for diabetes
A diabetes and disease technology, applied in pharmaceutical formulations, metabolic diseases, drug combinations, etc., can solve the problem of not knowing the treatment drugs for type II diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] [Example 1] Study on Effects on Insulin Resistance, Impaired Glucose Tolerance, and Instant Blood Glucose Levels in Mice Loaded with High Fat Meal
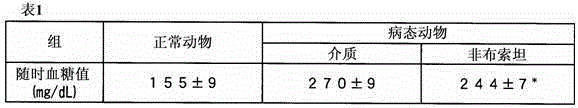
[0069] In order to study the effect of febuxostat (2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazole carboxylic acid) on insulin resistance, impaired glucose tolerance and blood glucose at any time, Febuxostat was administered to sick mice loaded with a high-fat diet and compared with the control group (vehicle group). In addition, in order to examine the relationship with the uric acid level in the blood, the uric acid level in the blood was measured.
[0070]
[0071] The 8-week-old male C57BL / 6J mice were loaded with a high-fat diet in which the proportion of calories from fat in the total calories (Fat Kcal%) was 60%. This leads to the onset of insulin resistance, impaired glucose tolerance and diabetes. For the febuxostat administration group, at the same time as the high-fat meal load was started, the tap water in...
Embodiment 2
[0101] [Example 2] Study on the Effect of Impaired Glucose Tolerance in Mice Loaded with High Fat Meal
[0102] To study the effect of febuxostat on impaired glucose tolerance, febuxostat was administered to sick mice loaded with a high-fat diet and compared with a control group (vehicle group).
[0103]
[0104]The 8-week-old male C57BL / 6J mice were loaded with a high-fat diet in which the proportion of calories from fat in the total calories (Fat Kcal%) was 60%. This leads to the onset of insulin resistance, impaired glucose tolerance and diabetes. For the febuxostat group, at the same time as the high-fat meal load started, the tap water with febuxostat dissolved in it was raised with the dose of 3 mg / kg / Day, and drinking water administration was carried out. Tap water for feeding.
[0105] At 12 weeks after the start of the high-fat meal load and febuxostat administration, a glucose load test was performed to evaluate impaired glucose tolerance. That is, 0.5 g / kg of g...
Embodiment 3
[0113] [Example 3] Administration of febuxostat preparations to patients with hyperuricemia
[0114] To study the effect of febuxostat preparations in patients with hyperuricemia whose blood uric acid value is above 7.0 mg / dL. Subjects were untreated diabetic patients aged 20 years or older, whose blood uric acid value was 7.0 mg / dL or higher, and who were not using urate-lowering drugs. Among them, patients with a presumed glomerular filtration rate of less than 30; patients with a history of hypersensitivity to febuxostat preparations; liver function (aspartate aminotransferase, and alanine aminotransferase) showed to be Patients who are more than 2 times the benchmark value of the drug implementing agency; patients with chronic liver disease, malignant tumor, active infection or inflammatory disease and other patients who are judged to be unsuitable by the attending doctor are excluded from the administration.
[0115] For this patient, febuxostat preparation 10 mg was adm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com