Enzyme directed immobilization method based on protein surface screening
A technology of immobilizing enzymes and immobilizing chambers, applied in immobilizing enzymes, biochemical equipment and methods, immobilizing on or in inorganic carriers, etc., can solve the problem of low sensitivity of enzyme test paper and enzyme biosensor, and uneven biofilm activity. , low remaining enzyme activity, etc., to achieve the effect of good enzyme activity maintenance, wide application range and large enzyme immobilization capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 - human erythrocyte catalase
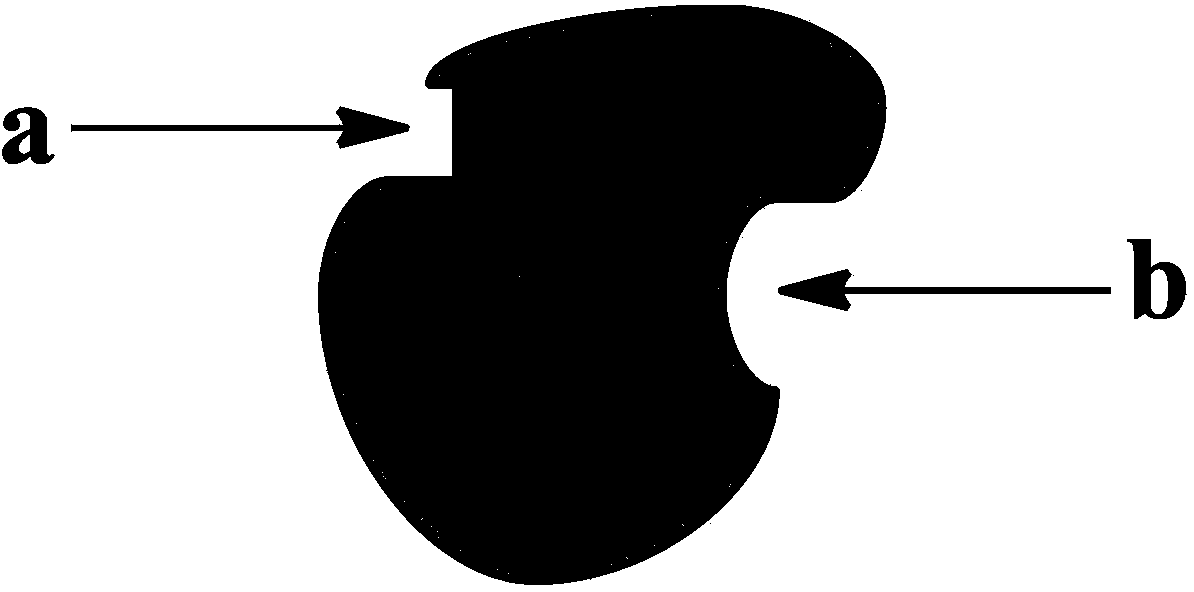
[0054] Human erythrocyte catalase (HUMAN ERYTHROCYTE CATALASE) was selected, and the three-dimensional crystal structure of human erythrocyte catalase molecular number 1DGB was used in the Protein Data Bank (Protein Data Bank, PDB) (the three-dimensional crystal structure was obtained from Protein Data Bank http: / / www .rcsb.org / pdb), analyzed the surface of human erythrocyte catalase by Sybyl software, and obtained that its catalytic center was located in the enzyme center, with the 147th asparagine (Asn147) and the 74th histidine (His74 ) midpoint as the center within range. Centered on the 176th leucine (Leu176) There are obvious depressions on the surface of human erythrocyte catalase protein in the range, which is far away from the catalytic center of human erythrocyte catalase, with a moderate volume, which can accommodate molecules with a molecular weight of 100-2000, and the interior of the depression is relatively ...
Embodiment 2
[0061] Example 2 - Catalase
[0062] Human erythrocyte catalase (HUMAN ERYTHROCYTE CATALASE) was selected, and the three-dimensional crystal structure of human erythrocyte catalase molecular number 1DGB was used in the Protein Data Bank (Protein Data Bank, PDB) (the three-dimensional crystal structure was obtained from Protein Data Bank http: / / www .rcsb.org / pdb), analyzed the surface of human erythrocyte catalase by Sybyl software, and obtained that its catalytic center was located in the enzyme center, with the 147th asparagine (Asn147) and the 74th histidine (His74 ) near the midpoint as the center within range. Centered around the 176th leucine (Leu176) There is a significant depression on the surface of human red blood cell catalase protein near the range, which is far away from the catalytic center of human red blood cell catalase, and has a moderate volume, which can accommodate molecules with a molecular weight of 100-2000, and the interior of the depression is re...
Embodiment 3
[0068] Example 3 - Acetylcholinesterase
[0069] Select acetylcholinesterase (Acetylcholine Esterase, AChE), using the three-dimensional crystal structure of human acetylcholinesterase molecule with PDB number 1F8U (the three-dimensional crystal structure comes from Protein Data Bank http: / / www.rcsb.org / pdb), through Sybyl software The surface analysis of acetylcholinesterase shows that its catalytic center is located in the enzyme center, with the 203rd serine (Ser203) as the center within range. Centered on the 400th aspartic acid (Asp400) There are obvious depressions on the surface of the acetylcholinesterase enzyme protein in the range, which is far away from the catalytic center of acetylcholinesterase, and has a moderate volume, which can accommodate molecules with a molecular weight of 100-2500, and the interior of the depression is relatively hydrophobic. Because the depression near the 400th aspartic acid (Asp400) has the above characteristics, it is selected as t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com