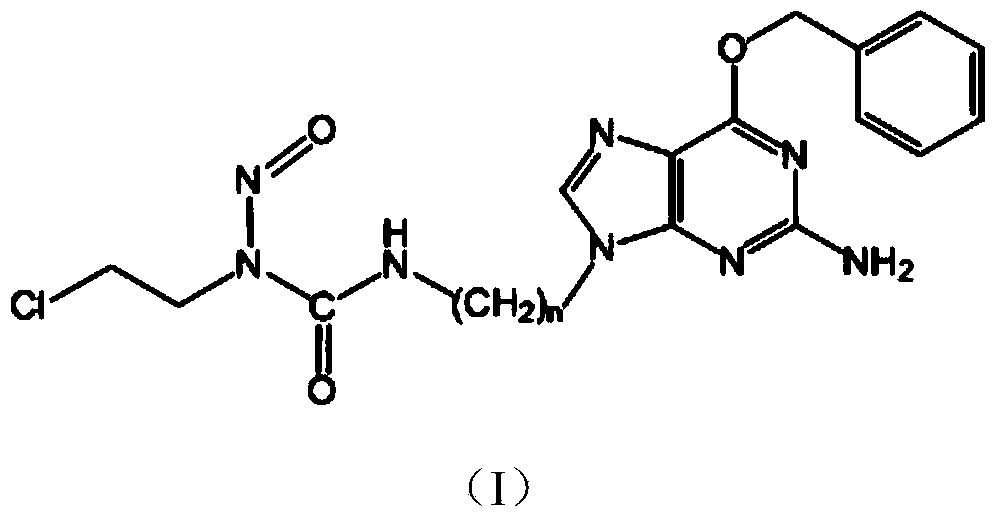
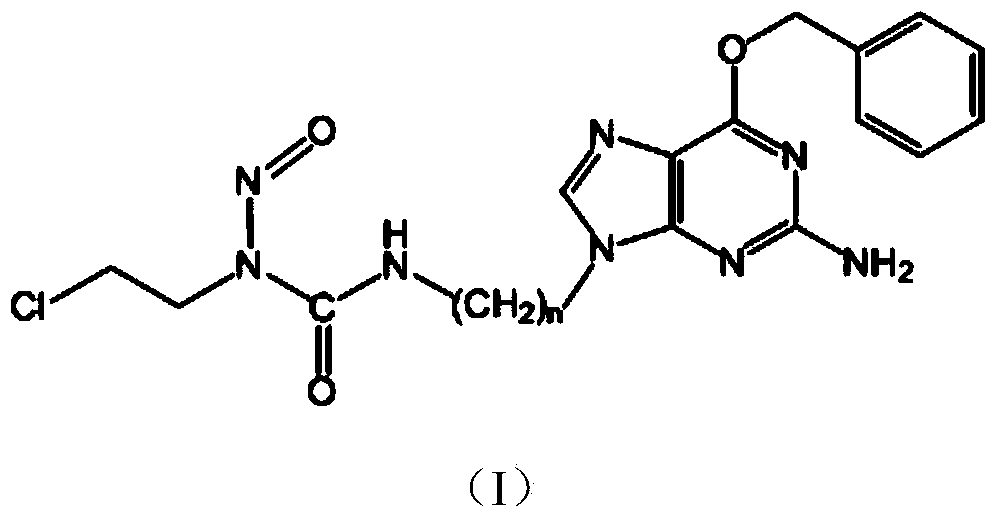
Novel chloroethyl nitrosourea with anticancer activity and synthesizing method thereof
An anti-cancer activity, nitrosourea technology, applied in the direction of organic active ingredients, organic chemistry, drug combination, etc., can solve the problems of reduced anti-cancer activity and high toxicity, achieve low drug resistance and improve targeting , The effect of high anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
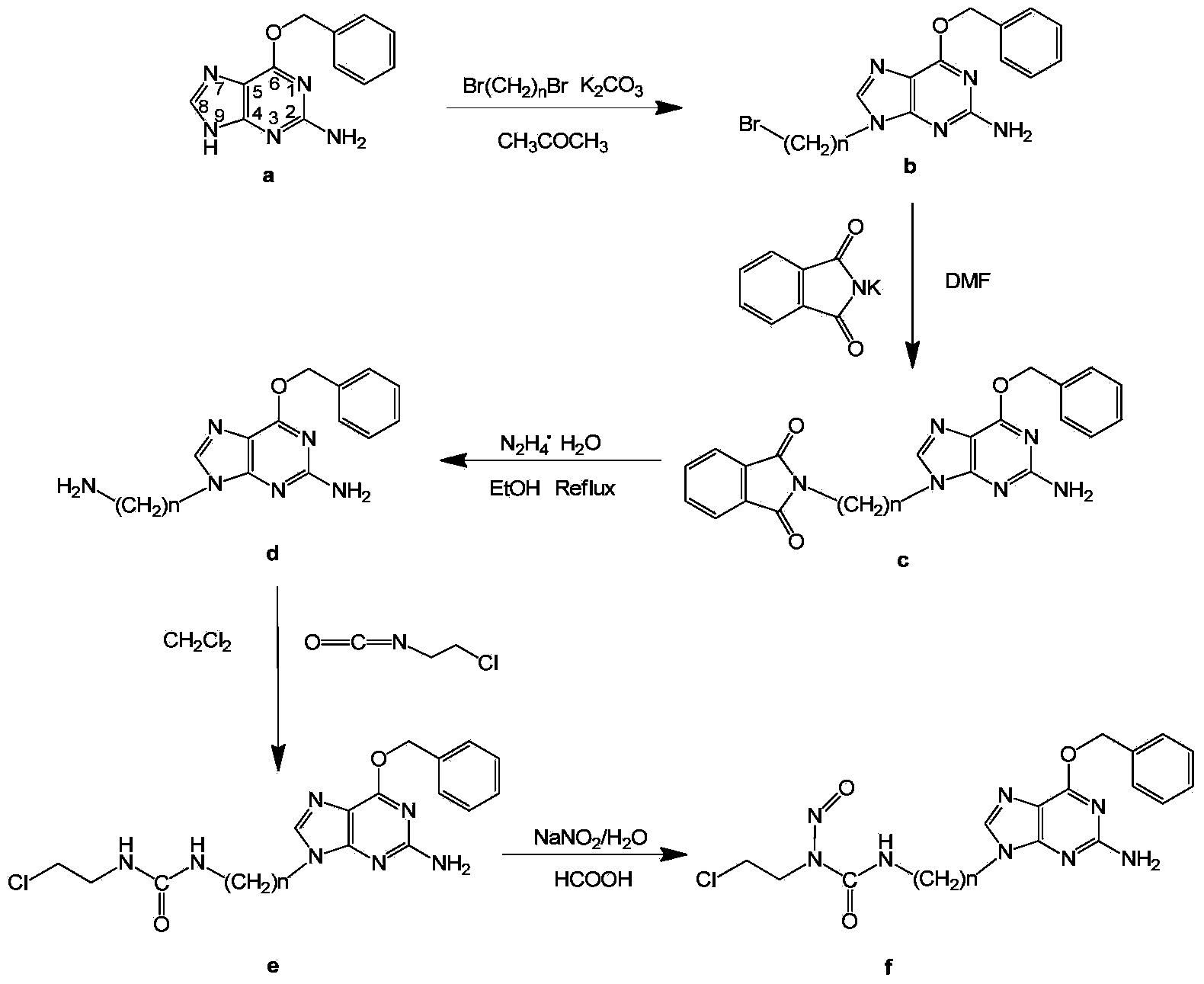
[0071] Embodiment 1: N-(2-chloroethyl)-N'-3-(O 6 Synthesis of -Benzyl-9-guanyl)ethyl-N-nitrosourea (compound 1)
[0072] 1) N9-Bromoethyl-O 6 -Synthesis of benzylguanine
[0073] Weigh O 6 -Benzylguanine (0.96g, 4mmol), anhydrous potassium carbonate (1.66g, 12mmol) were added to a three-necked flask, 100mL of acetone was added, the temperature was slowly raised to 50°C, and 1,2-dibromoethane (3.10 g, 16mmol), continue to react for 48h after dropping, monitor the progress of the reaction by thin-layer chromatography, filter to the point where there is no raw material, collect the filtrate, distill the solvent under reduced pressure at 40°C, and dissolve the obtained yellow oil in 2mL Methanol was separated and purified by silica gel column chromatography. The eluents were petroleum ether and ethyl acetate. Gradient elution was adopted. The volume ratio of petroleum ether / ethyl acetate was gradually increased from 2:1 to 5:1. Vacuum at 60°C Drying gave white solid N9-bromoet...
Embodiment 2
[0096] Embodiment 2: N-(2-chloroethyl)-N'-3-(O 6 Synthesis of -Benzyl-9-guanyl)propyl-N-nitrosourea (Compound 2)
[0097] 1) N9-bromopropyl-O 6 -Synthesis of benzylguanine
[0098] Weigh O 6 -Benzylguanine (0.48g, 2mmol), anhydrous potassium carbonate (0.55g, 4mmol) were added to a three-necked flask, 50mL of acetone was added, the temperature was slowly raised to 40°C, and 1,3-dibromopropane (1.62g ,8mmol), continue to react for 72h after dropping. Thin-layer chromatography was used to monitor the degree of progress of the reaction, and to the point where there was no raw material, it was filtered. Collect the filtrate, spin the solvent under reduced pressure at 35°C, dissolve the obtained yellow oil in 1 mL of methanol, and separate and purify it by silica gel column chromatography. The eluents are petroleum ether and ethyl acetate, and gradient elution is used. The volume ratio of ethyl acetate / ethyl acetate gradually increased from 2:1 to 5:1. Vacuum drying at 60°C ga...
Embodiment 3
[0121] Embodiment 3: N-(2-chloroethyl)-N'-3-(O 6 Synthesis of -Benzyl-9-guanyl)ethyl-N-nitrosourea (Compound 3)
[0122] 1) N9-bromobutyl-O 6 -Synthesis of benzylguanine
[0123] Weigh O 6 -Benzylguanine (1.93g, 8mmol), anhydrous potassium carbonate (2.21g, 16mmol) were added to a three-necked flask, 150mL of acetone was added, the temperature was slowly raised to 60°C, and 1,4-dibromobutane (6.91 g, 32mmol), continue to react for 40h after dropping, and monitor the progress of the reaction by thin-layer chromatography, and filter after no raw material point. The filtrate was collected, and the solvent was spin-dried under reduced pressure at 40°C. The obtained yellow oil was dissolved in 4 mL of methanol, and separated and purified by silica gel column chromatography. The volume ratio of ethyl ester was gradually increased from 2:1 to 5:1, and dried under vacuum at 60°C to obtain white solid N9-bromobutyl-O 6 - Benzylguanine (1.35 g, 5.0 mmol), yield 63%.
[0124] UV la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com