Green synthesis method for benzoin
A technology of green synthesis and benzoin, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of low catalytic efficiency, large dosage, and difficulty in catalyst synthesis, and achieve simple process and rich sources of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthesis of embodiment 1 imidazole hydrochloride
[0023] Add 68g of imidazole into the four-necked flask, dissolve it in 100ml of tetrahydrofuran, slowly add 85ml of 37% hydrochloric acid solution dropwise to the flask through the dropping funnel, keep the reaction temperature at 60-70°C, and put it into an ice-water bath to crystallize after reacting for 1h. Filtrate and dry to obtain imidazole hydrochloride, and obtain 98.6 g of imidazole hydrochloride with a yield of about 95%.
Embodiment 2-1
[0024] The synthesis of embodiment 2-1 benzoin
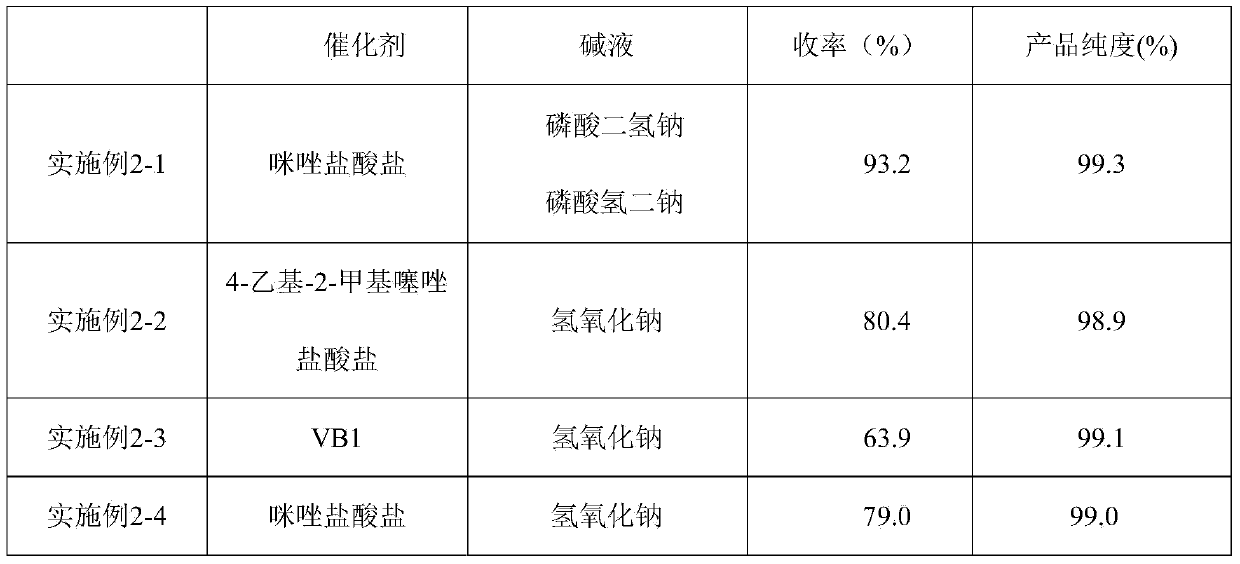
[0025] Add 106g of raw materials benzaldehyde, 1.0g of imidazole hydrochloride, 72g of absolute ethanol, a buffer solution of sodium dihydrogen phosphate and disodium hydrogen phosphate in a certain ratio, adjust the pH value to 7.8-8.5, and react at 80°C 2h. Put it into an ice-water bath for crystallization and filtration to obtain a light yellow benzoin solid, which was washed with a small amount of ethanol to obtain white needle-shaped crystals and dried under normal pressure to obtain 98.8 g of the finished product.
Embodiment 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com