Electrochemical sensor for detecting heavy metals and preparation method and application thereof
An electrochemical and sensor technology, applied in the field of sensors, can solve the problems of inability to monitor online, limit wide application, and low detection limit, and achieve the effects of fast production, improved electrode response, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: electrochemical sensor and preparation method thereof
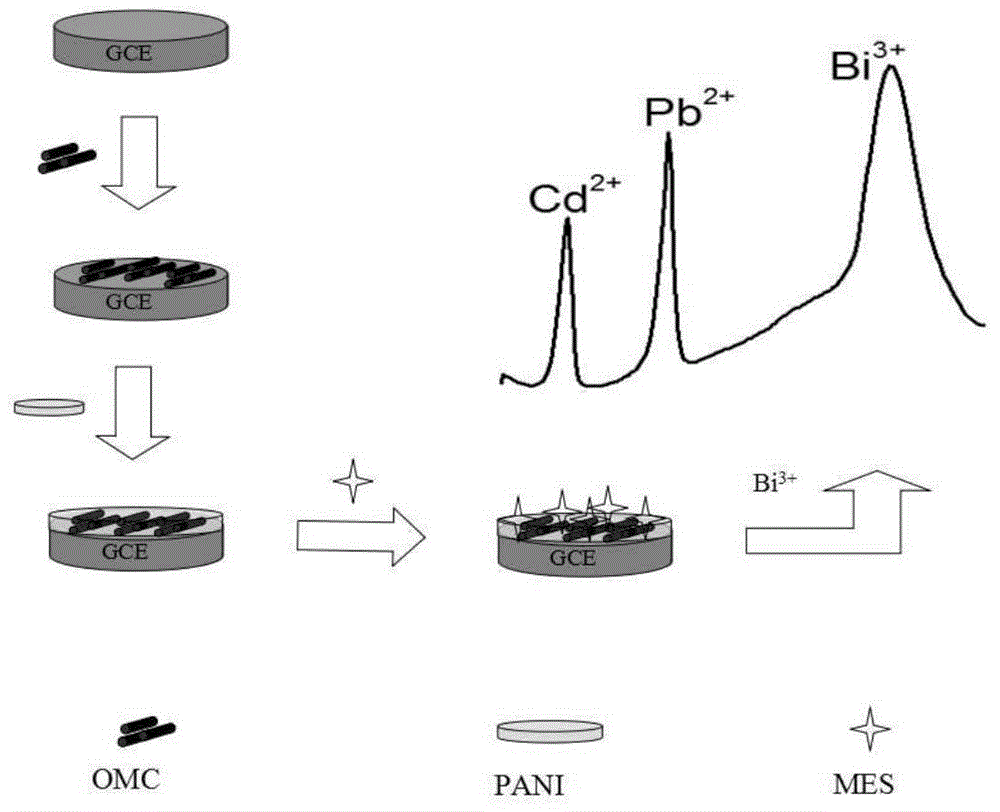
[0048] see figure 1 , an electrochemical sensor, including a glassy carbon electrode, the surface of the glassy carbon electrode is modified with a layer of ordered mesoporous carbon, a layer of polyaniline is electrodeposited on the ordered mesoporous carbon, and 2-mercapto is electrodeposited on the polyaniline Sodium ethanesulfonate.
[0049] The preparation method of the aforementioned electrochemical sensor specifically comprises the following steps:
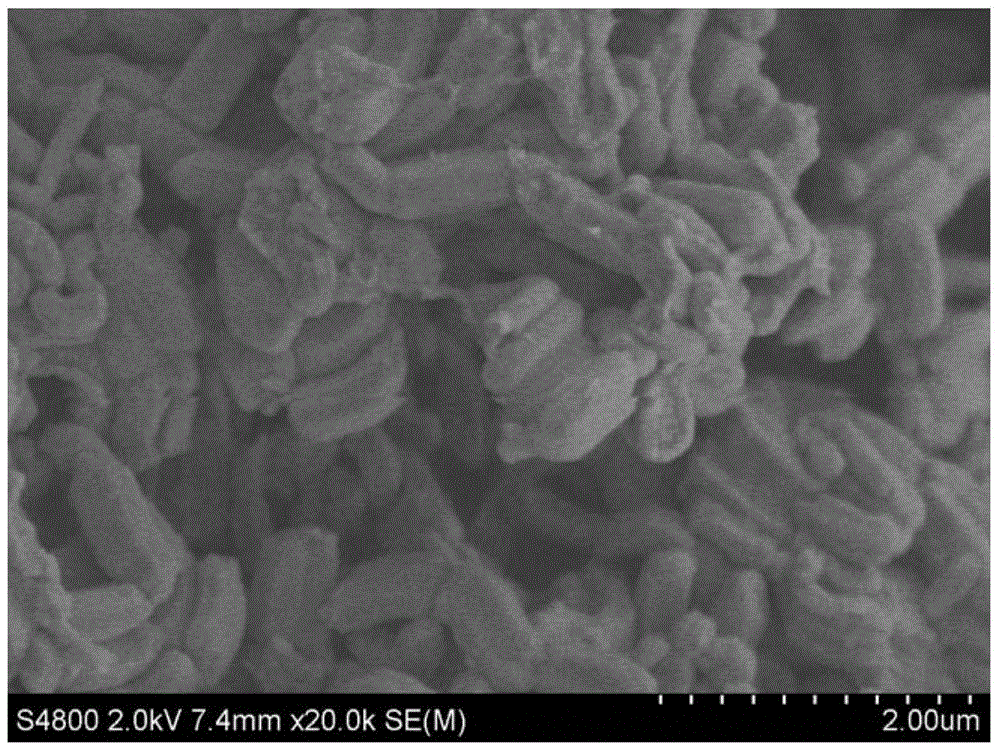
[0050] 1. Preparation of ordered mesoporous carbon:
[0051] (1) Synthesis of mesoporous silicon template SBA-15: 8.0g block copolymer P123 is placed in hydrochloric acid solution (hydrochloric acid solution is made of 270g H 2 O and 320mL, 1.54M hydrochloric acid mixed), stirred in a 35°C water bath (water bath temperature can be 30-35°C) until the block copolymer P123 was dissolved, and then added 17g of ethyl orthosilicate dropwise The ester...
Embodiment 2
[0072] Embodiment 2: the application method of electrochemical sensor
[0073]The glassy carbon electrode of Example 1 is used as a working electrode, a saturated calomel electrode is used as a reference electrode, and a platinum electrode is used as a counter electrode to establish a three-electrode system. The electrolyte solution in the electrolytic cell of the three-electrode system is 0.1 μM of bismuth ions M, pH 4.5 acetate buffer solution (the electrolyte solution may also be 0.1M pH 3-6 acetate buffer containing 0.5-1.5 μM bismuth ions). The three-electrode system was connected to an electrochemical workstation, and the concentration of heavy metal ions in the solution to be tested was detected by differential pulse anodic stripping voltammetry.
[0074] The working process of differential pulse anode stripping voltammetry: pre-enrich the heavy metal ions in the solution to be tested on the working electrode, the pre-enrichment voltage is -1.2V, and the pre-enrichment ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com