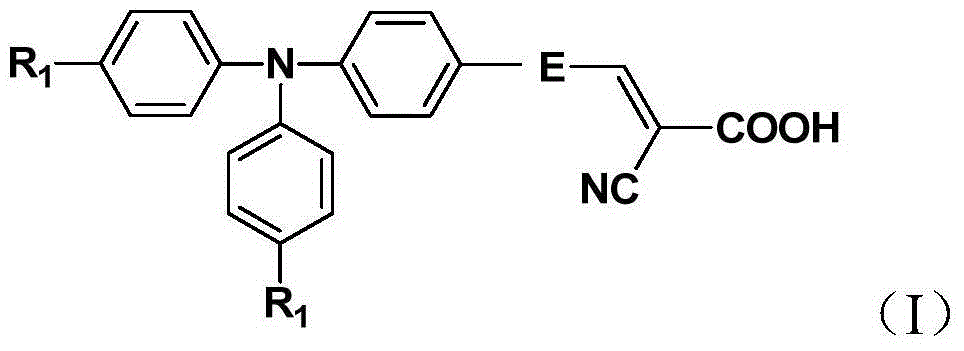
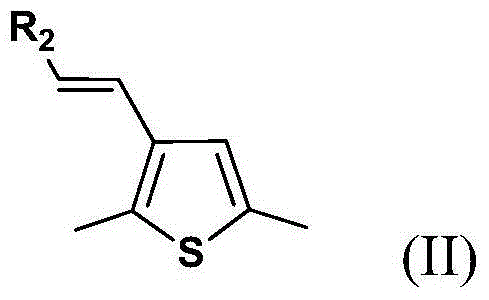
Organic dye sensitizer and preparation method thereof
A technology of organic dyes and sensitizers, applied in the directions of organic dyes, chemical instruments and methods, photosensitive equipment, etc., can solve the problems of yield and energy conversion efficiency to be improved, high cost, cumbersome and other problems, and achieve good practical application value, The effect of low production cost and common and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
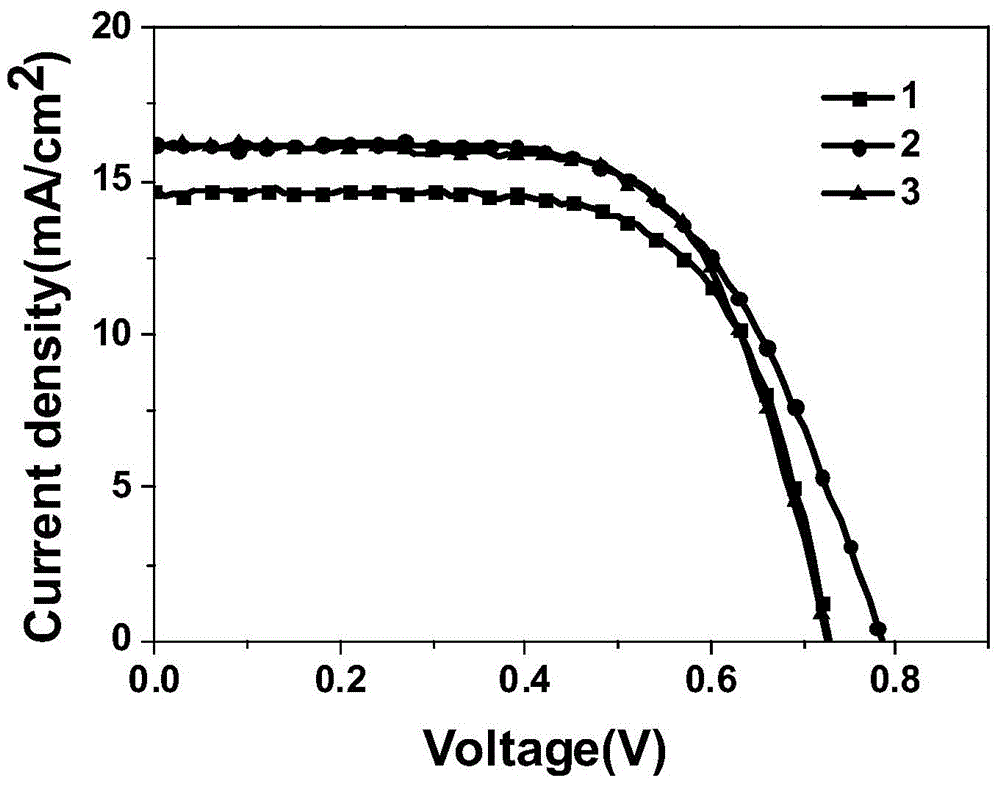
Embodiment 1
[0046] A kind of organic dye sensitizer whose chemical structural formula is 1, its synthetic route is as follows:
[0047]
[0048] (1) chemical structural formula is the synthesis of the intermediate of b:
[0049] Add compound a (0.50g, 1.6mmol), compound 1-hexenal (0.27g, 2.7mmol), and 25mL THF into a 100mL two-neck flask. Stir for half an hour under the protection of nitrogen, and then slowly dropwise add a THF solution in which 0.25 g (2.2 mmol) of potassium tert-butoxide is dissolved. Stir for 1 hour after dropping, and then slowly raise the temperature to 50°C. React for 12 hours. After cooling to room temperature, it was extracted with dichloromethane, followed by MgSO 4 Dry, filter and spin dry. Use petroleum ether as the eluent to pass through a silica gel column. A light yellow liquid was obtained with a yield of 68%. 1 H NMR (400MHz, CDCl 3 ,δ / ppm):7.20-7.19(d,1H,J=5.17Hz),7.09-7.08(d,1H,J=6.00Hz),6.65-6.58(m,1H),6.45-6.41(m,1H ),6.24-6.18(m,1H),5.87-5....
Embodiment 2
[0059] The synthetic route of the organic dye sensitizer whose chemical structural formula is 2 is as follows:
[0060]
[0061] (1) chemical structural formula is the synthesis of the intermediate of b:
[0062] Add compound a (0.50g, 1.6mmol), compound 1-hexenal (0.27g, 2.7mmol), and 25mL THF into a 100mL two-neck flask. Stir for half an hour under the protection of nitrogen, and then slowly dropwise add a THF solution in which 0.25 g (2.2 mmol) of potassium tert-butoxide is dissolved. Stir for 1 hour after dropping, and then slowly raise the temperature to 50°C. React for 12 hours. After cooling to room temperature, it was extracted with dichloromethane, followed by MgSO 4 Dry, filter and spin dry. Use petroleum ether as the eluent to pass through a silica gel column. A light yellow liquid was obtained with a yield of 68%. 1 H NMR (400MHz, CDCl 3 ,δ / ppm):7.20-7.19(d,1H,J=5.17Hz),7.09-7.08(d,1H,J=6.00Hz),6.65-6.58(m,1H),6.45-6.41(m,1H ),6.24-6.18(m,1H),5.87-5.80(m...
Embodiment 3
[0072] The synthetic route of the organic dye sensitizer whose chemical structural formula is 3 is as follows:
[0073]
[0074] (1) chemical structural formula is the synthesis of the intermediate of i:
[0075] Add compound a (0.50g, 1.6mmol), compound 5-hexylthiophene 2-carbaldehyde (0.53g, 2.7mmol), 25mL THF into a 100mL two-necked flask. Stir for half an hour under the protection of nitrogen, and then slowly dropwise add 30 mL of a THF solution in which 0.27 g (2.4 mmol) of potassium tert-butoxide is dissolved. Stir for 1 hour after dropping, and then slowly raise the temperature to 50°C. React for 24 hours. After cooling to room temperature, it was extracted with dichloromethane, followed by MgSO 4 Dry, filter and spin dry. Use petroleum ether as the eluent to pass through a silica gel column. A yellow liquid was obtained with a yield of 64%. 1 H NMR (400MHz, CDCl 3 ,δ / ppm):7.26(s,1H),7.18-7.16(d,1H,J=5.32Hz),7.07-7.05(d,1H,J=7.98Hz),6.90(s,1H),6.81- 6.79(d,1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com