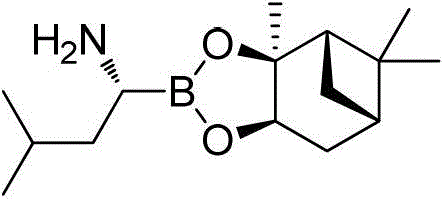
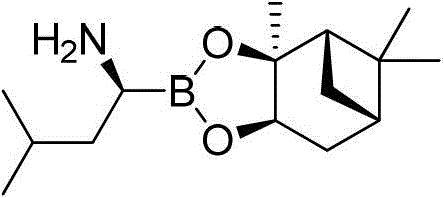
Amide boronic acid ester for detecting the purity of bortezomib intermediate, preparation method and application thereof
An amide boronate and leucine boronate technology, applied in the field of medicine, can solve the problems of interference analysis and detection, instability, and inability to detect the optical purity of products, and achieve high optical purity, short reaction time, sensitivity and accuracy. high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the synthesis of formula B compound:
[0057] The compound of formula A1 (200mg, 0.8mmol, 1eq) was weighed and placed in a 25mL single-necked bottle. The temperature was cooled in an ice bath, and regular dichloromethane (8 mL) was added. Then p-bromobenzoyl chloride (263mg, 1.2mmol, 1.5eq) was weighed and added to the reaction flask. Triethylamine (0.22 mL, 1.6 mmol, 2.0 eq) was measured and added dropwise to the reaction. The reaction lasted for about 10 minutes, and when the reaction was over, a white solid precipitated out, and 244 mg of the white solid was obtained by filtration, and another 70 mg was obtained by column chromatography of the mother liquor, which was identified as the compound of formula B1 by 1HNMR and ESI-MS, and the reaction was quantitatively completed.
[0058] R f =0.4(PE / AE=2:1)
[0059] 1 HNMR (CDCl 3 ,400MHz):δ7.65(d,J=8.4Hz,2H),7.56(d,J=8.4Hz,2H),7.04(brs,1H),3.08-3.02(m,1H),1.73-1.64( m,1H),1.52(t,J=7.2Hz,2H),1.27(s,6H)...
Embodiment 2
[0061] Embodiment 2: the synthesis of formula IIb+II'b mixture:
[0062] Weigh the compound of formula B1 (317mg, 0.80mmol, 1eq) and dissolve it in THF (4mL), add (+)-pinanediol (272mg, 1.60mmol, 2eq), and react at room temperature for 2 hours to obtain IIb+II'b mixture (230mg, 64%).
[0063] 1 HNMR (CDCl 3 ,400MHz):δ7.64,7.63(d,J=8.0Hz,2H),7.55(d,J=8.4Hz,2H),6.70,6.59(brs,1H),4.36-4.27(m,1H), 3.36-3.19(m,1H),2.40-2.28(m,1H),2.26-2.13(m,1H),2.09-1.97(m,1H),1.95-1.80(m,2H),1.78-1.61(m ,1H),1.61-1.51(m,2H),1.44,1.42(s,3H),1.41-1.33(m,1H),1.29(s,3H),0.95(d,J=6.4Hz,6H), 0.86(s,3H).
[0064] MS(ESI,m / z):446.1([M( 79 Br)-H] + ),448.1([M( 81 Br)-H] + ).
Embodiment 3
[0065] Embodiment 3: the synthesis of formula IIb compound:
[0066] The compound of formula Ib (90mg, 0.3mmol, 1eq) was weighed and placed in a 25mL single-necked bottle. The temperature was cooled in an ice bath, and regular dichloromethane (3 mL) was added. Then weigh p-bromobenzoyl chloride (99mg, 0.45mmol, 1.5eq) and add it into the reaction flask. Measure Et 3 N (84 μL, 0.6 mmol, 2.0 eq), was added dropwise to the reaction. The reaction was about 5 minutes, and the reaction ended. Direct concentration, dry loading, column chromatography. The target product (133 mg, yield 99%, ee value 97.0%) was obtained.
[0067] 1 HNMR (CDCl 3 ,400MHz):δ7.64(d,J=8.4Hz,2H),7.56(d,J=8.4Hz,2H),6.64(brs,1H),4.34-4.29(m,1H),3.29-3.21( m,1H),2.40-2.29(m,1H),2.22-2.13(m,1H),2.07-1.99(m,1H),1.95-1.82(m,2H),1.78-1.67(m,1H), 1.59-1.51(m,2H),1.44(s,3H),1.38-1.33(m,1H),1.29(s,3H),0.95(d,J=6.8Hz,6H),0.86(s,3H) .
[0068] MS(ESI,m / z):446.1([M( 79 Br)-H] + ),448.1([M( 81 Br)-H] + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com