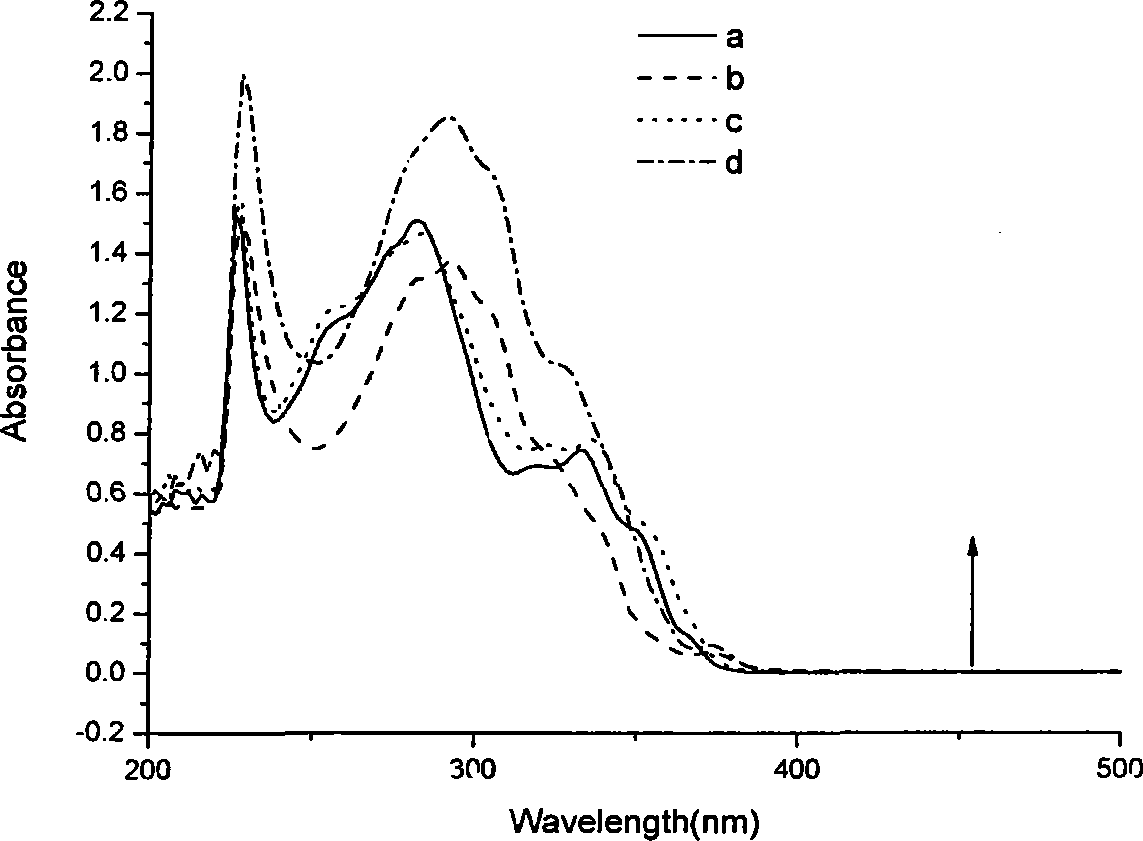
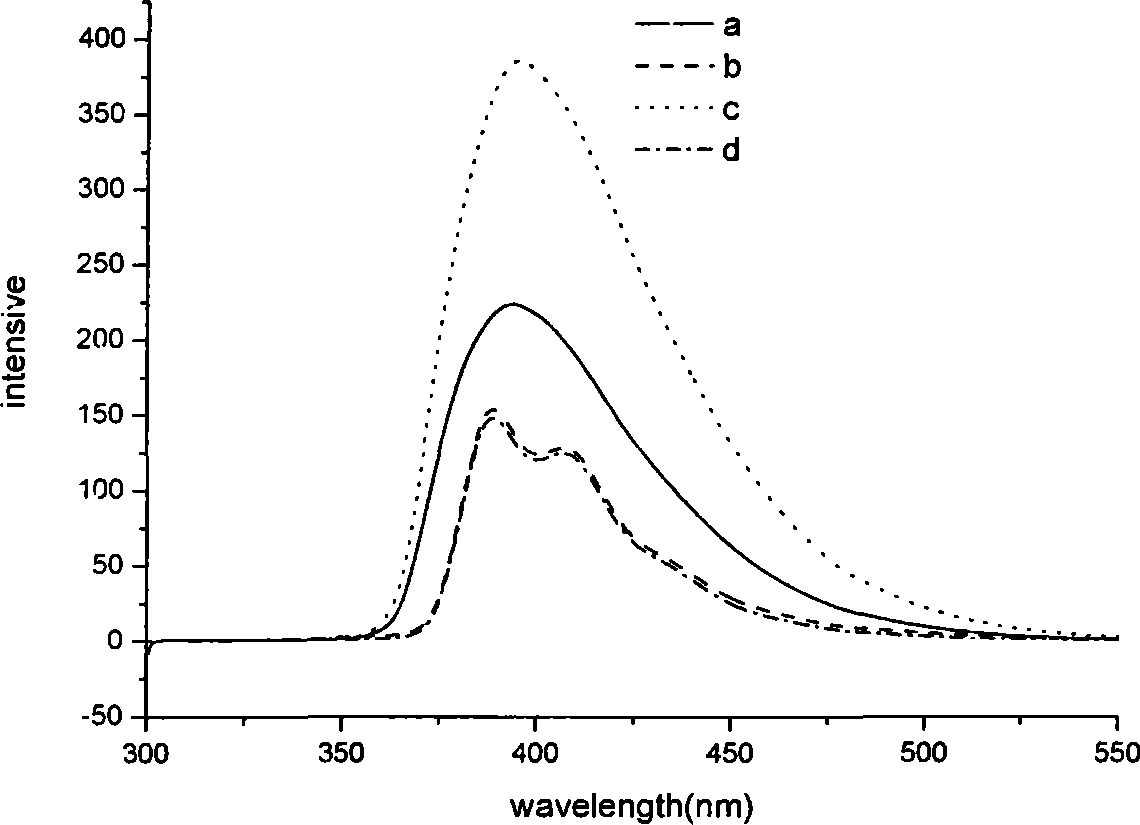
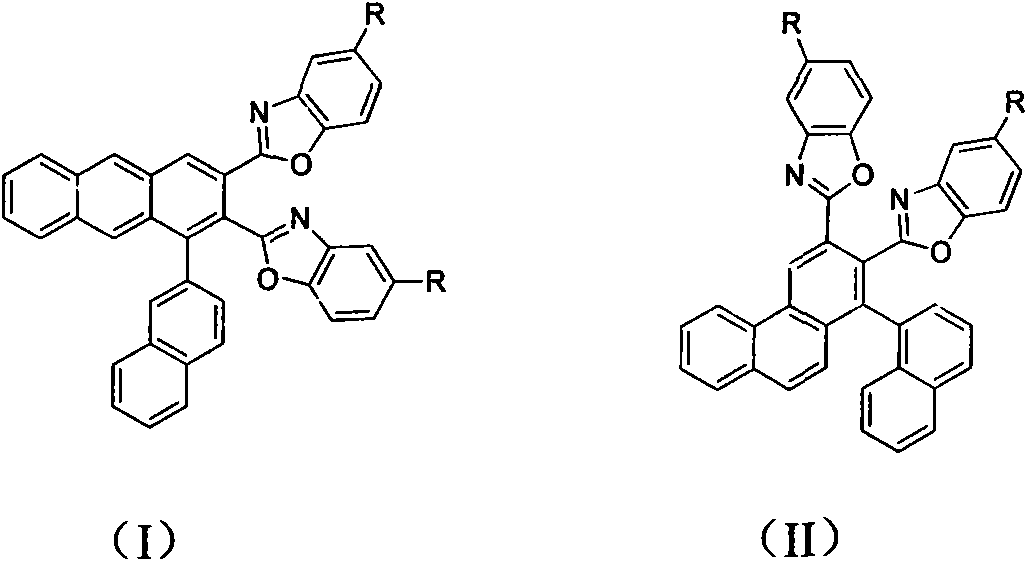
Blue ray material with triaryl anthracene or triaryl phenanthrene structure and preparation method of blue ray material
A technology of triarylphenanthrene and blue light materials, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of device performance influence, film loosening, etc., to enhance compatibility, enhance luminous intensity, reduce The effect of the quenching effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, the structure and synthesis of 1-(2-naphthyl)-2,3-bis(2-benzoxazolyl)anthracene
[0025]
[0026] Weigh 400mg (1.48mmol) of 1-(2-naphthyl)-2-(2-benzoxazolyl)acetylene in an internal immersion photoreactor, add 80ml of acetonitrile, and ultrasonically dissolve it completely, the solution is colored liquid and then sealed. Under a nitrogen protection environment, a 300W medium-pressure mercury lamp was used to illuminate, and the reaction was monitored by TLC at any time. After 2 hours, it was found that only a new point was generated, and the light was continued, and the color of the solution gradually became darker. After 6 hours, the basic reaction of the raw materials was completed, and the reaction was stopped. The solvent was spin-dried to obtain a tan solid, which was separated by 100-200 mesh silica gel column chromatography, and the precipitant was dichloromethane: ethyl acetate = 1: 1 to obtain 260 mg of white powder with a yield of 65%, m.p.: ...
Embodiment 2
[0027] Example 2. The structure and synthesis of 1-(1-naphthyl)-2,3-bis(2-benzoxazolyl)phenanthrene
[0028]
[0029] 1.00 g of 1-(1-naphthyl)-2-(2-benzoxazolyl)acetylene was dissolved in 150 ml of acetonitrile to obtain a clear light yellow solution. After adopting a 300W medium-pressure mercury lamp to irradiate for 6 hours, it was found by spotting that the reaction was substantially complete, and the acetonitrile solvent was spin-dried, and column chromatography was carried out with PE: EA: DCM=90: 1: 1 to obtain 0.721g of pure product, and the yield was 72%, m.p.: 224-228°C, 1 H NMR (400MHz, CDCl 3 , 25°C, TMS): δ9.93(s, 1H, J=8.4Hz), 9.10-9.08(d, 1H, J=8.4Hz), 7.2-7.90(m, 1H), 7.55-7.40(m, 6H), 7.34-7.30 (m, 1H), 7.26-7.12 (m, 6H) ppm; 13 C NMR (400MHz, CDCl 3 , 25°C, TMS): 6161.1, 160.5, 149.9, 149.7, 141.2, 140.9, 140.3, 133.7, 132.3, 132.2, 132.1, 131.7, 130.6, 129.1, 128.0, 127.7, 127.7, 1267.3, 1257.2, 12 , 124.5, 124.2, 124.1, 124.0, 124.0, 123.8, 123.7, 12...
Embodiment 3
[0030] Example three, the structure and synthesis of 1-(2-naphthyl)-2,3-bis(5-tert-butyl-2-benzoxazolyl)anthracene
[0031]
[0032]Using 1-(2-naphthyl)-2-(5-tert-butyl-2-benzoxazolyl)acetylene as raw material, it was prepared under the same reaction conditions as in Example 1. Yield 70%, m.p.: 232-234°C; 1 H NMR (400MHz, CDCl 3 , 25°C, TMS): δ (ppm) 9.01 (s, 1H), 7.98 (d, 1H, J=8.8Hz), 7.93-7.86 (m, 3H), 7.81-7.77 (m, 2H), 7.68 ( d, 1H, J=7.6Hz), 7.55-7.53(m, 3H), 7.46-7.38(m, 4H), 7.28(s, 2H), 7.24(m, 4H), 1.31(d, 18H, J= 2Hz); 13 CNMR (400MHz, CDCl 3 ,25℃,TMS):δ(ppm)161.59,161.40,148.77,148.64,147.99,147.09,142.89,141.59,141.34,138.16,134.52,134.43,133.39,132.53,130.67,130.42,130.34,130.21,128.83,128.74 , 128.46, 128.27, 127.94, 127.70, 127.27, 127.11, 127.05, 126.13, 126.04, 125.07, 123.06, 122.37, 116.67, 116.43, 109.38, 109.35, 364.84, 3114.82, 3MS (IES + )[M+H] + calculated for C 46 h 39 N 2 o 2 : 651.3012, found: 651.3016.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com