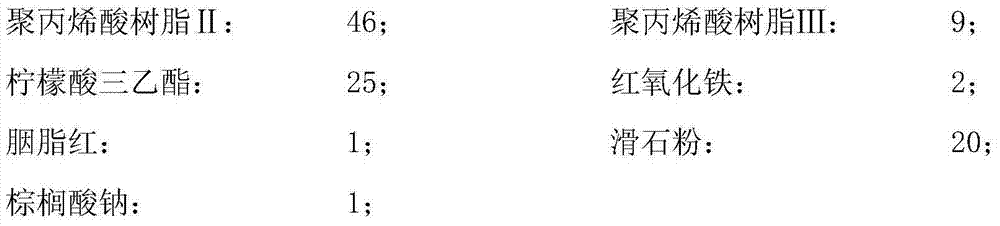Orally delivered intestinal site-specific drug release film coating premixed auxiliary material and preparation method thereof
A technology of film coating and premixing auxiliary materials, which is applied in the directions of pharmaceutical formulations, drug delivery, and medical preparations with non-active ingredients, etc., can solve the problems of low production efficiency, high production cost, and difficult operation in the coating process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: A premixed auxiliary material for oral administration of enteric localized drug release film coating, when the pH value of the intestinal tract is 5.0, the localized drug release film coating premixed auxiliary material consists of the following raw materials in parts by weight:
[0056] Polyacrylic resin Ⅱ: 54; Polyacrylic resin Ⅲ: 3;
[0057] Triethyl citrate: 20; Titanium dioxide: 1;
[0058] Talc: 8; Sodium Palmitate: 2;
[0059] Wherein, the viscosity of the polyacrylic resin II is 18 mPa·s, and the viscosity of the polyacrylic resin III is 17 mPa·s.
[0060] The preparation method of the above-mentioned oral administration enteric localized drug release film coating premixed auxiliary material comprises the following steps:
[0061] S1. Weighing: Accurately weigh each raw material according to the above ratio, and set aside;
[0062] S2. Premixing: Put the above-mentioned weighed raw materials into a ziplock bag and mix for 5 minutes;
[0063] S3...
Embodiment 2
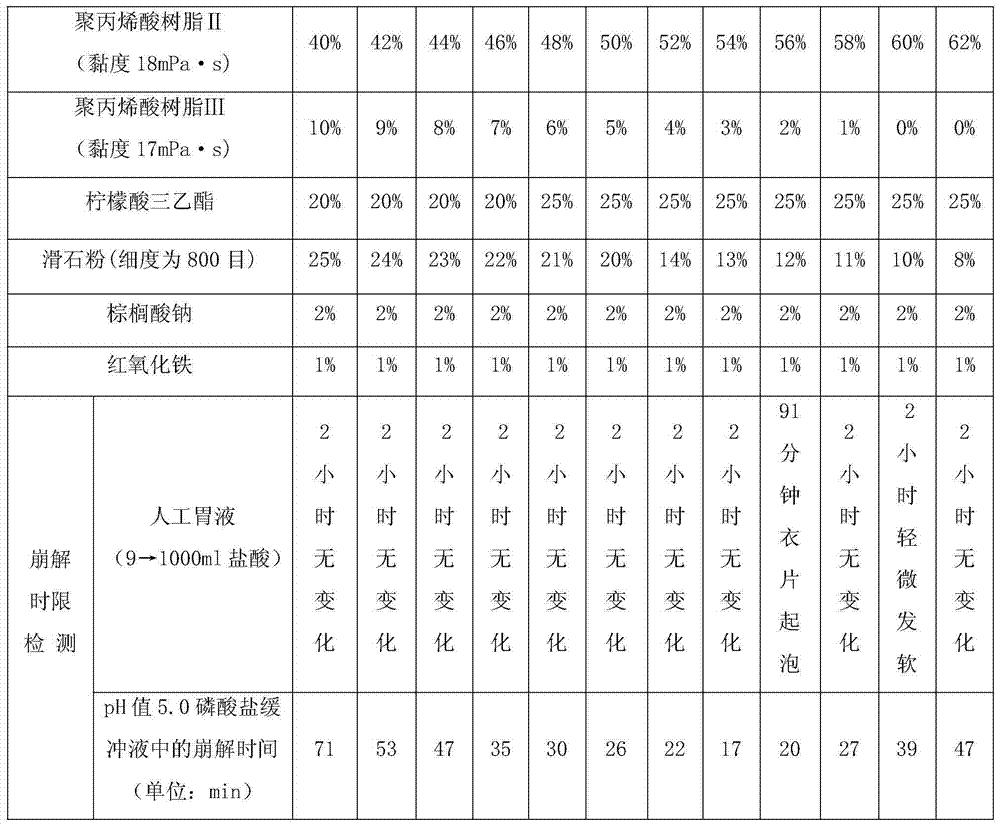
[0066] Embodiment 2: A premixed auxiliary material for oral administration of enteric localized drug release film coating, when the pH value of the intestinal tract is 5.8, the localized drug release film coating premixed auxiliary material consists of the following raw materials in parts by weight:
[0067]
[0068] Wherein, the viscosity of the polyacrylic resin II is 18 mPa·s, and the viscosity of the polyacrylic resin III is 17 mPa·s.
[0069] The preparation method of the above-mentioned oral administration enteric localized drug release film coating premixed auxiliary material comprises the following steps:
[0070] S1. Weighing: Accurately weigh each raw material according to the above ratio, and set aside;
[0071] S2. Premixing: put the above-mentioned weighed raw materials into a ziplock bag and mix for 10 minutes;
[0072] S3. Pulverization: put the premixed raw materials into an ultrafine pulverizer and grind them for 150 minutes, and pass the ground raw materi...
Embodiment 3
[0075] Embodiment 3: A premixed auxiliary material for oral administration of enteric localized drug release film coating. When the pH value of the intestinal tract is 6.8, the localized drug release film coating premixed auxiliary material consists of the following raw materials in parts by weight:
[0076] Polyacrylic resin Ⅱ: 44; Polyacrylic resin Ⅲ: 13;
[0077] Triethyl citrate: 25; Tartrazine: 3;
[0078] Talc: 13; Sodium Palmitate: 2;
[0079] Wherein, the viscosity of the polyacrylic resin II is 18 mPa·s, and the viscosity of the polyacrylic resin III is 26 mPa·s.
[0080] The preparation method of the above-mentioned oral administration enteric localized drug release film coating premixed auxiliary material comprises the following steps:
[0081] S1. Weighing: Accurately weigh each raw material according to the above ratio, and set aside;
[0082] S2. Premixing: Put the above-mentioned weighed raw materials into a ziplock bag and mix for 6 minutes;
[0083] S3. Pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com