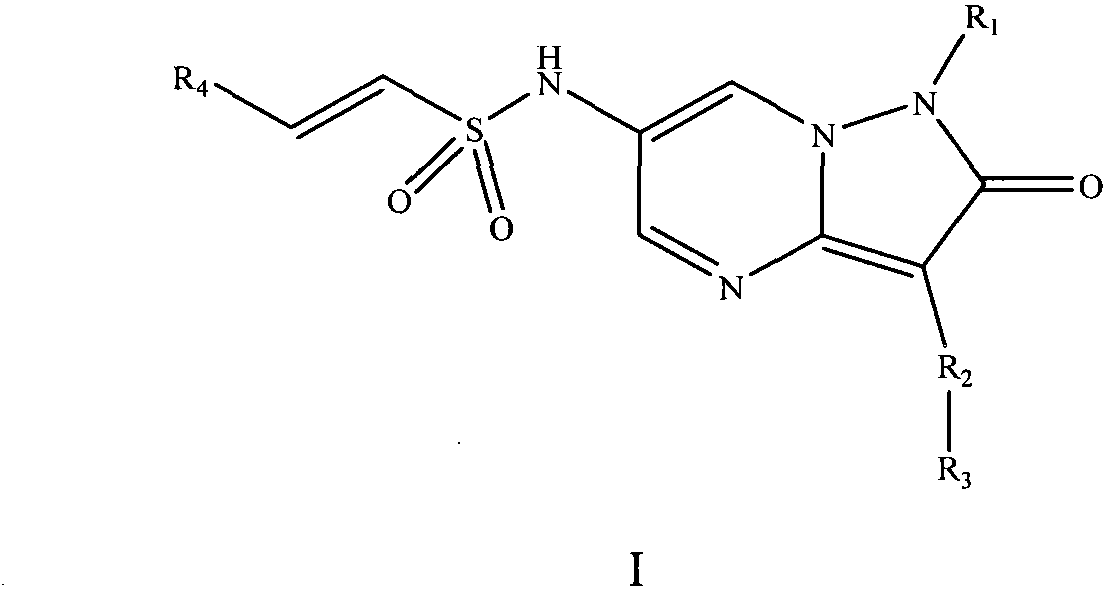
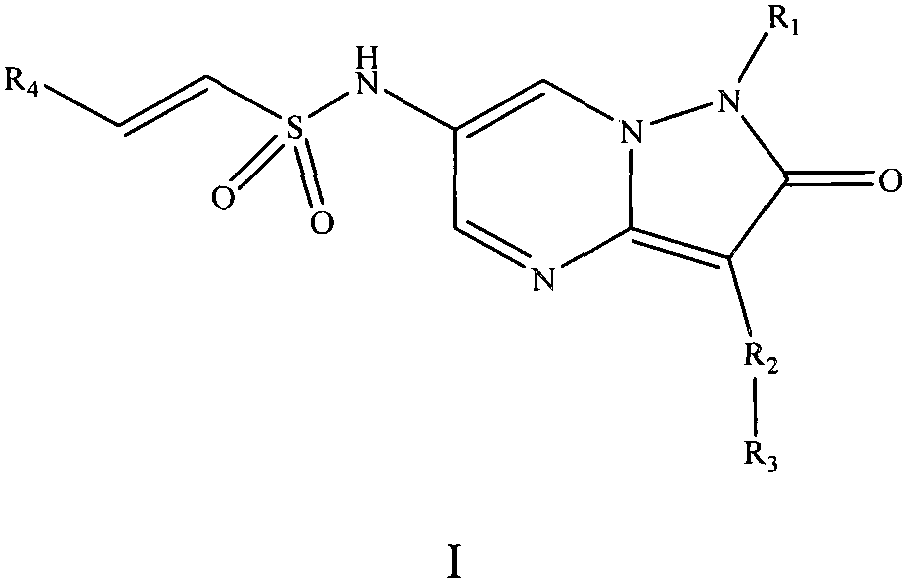
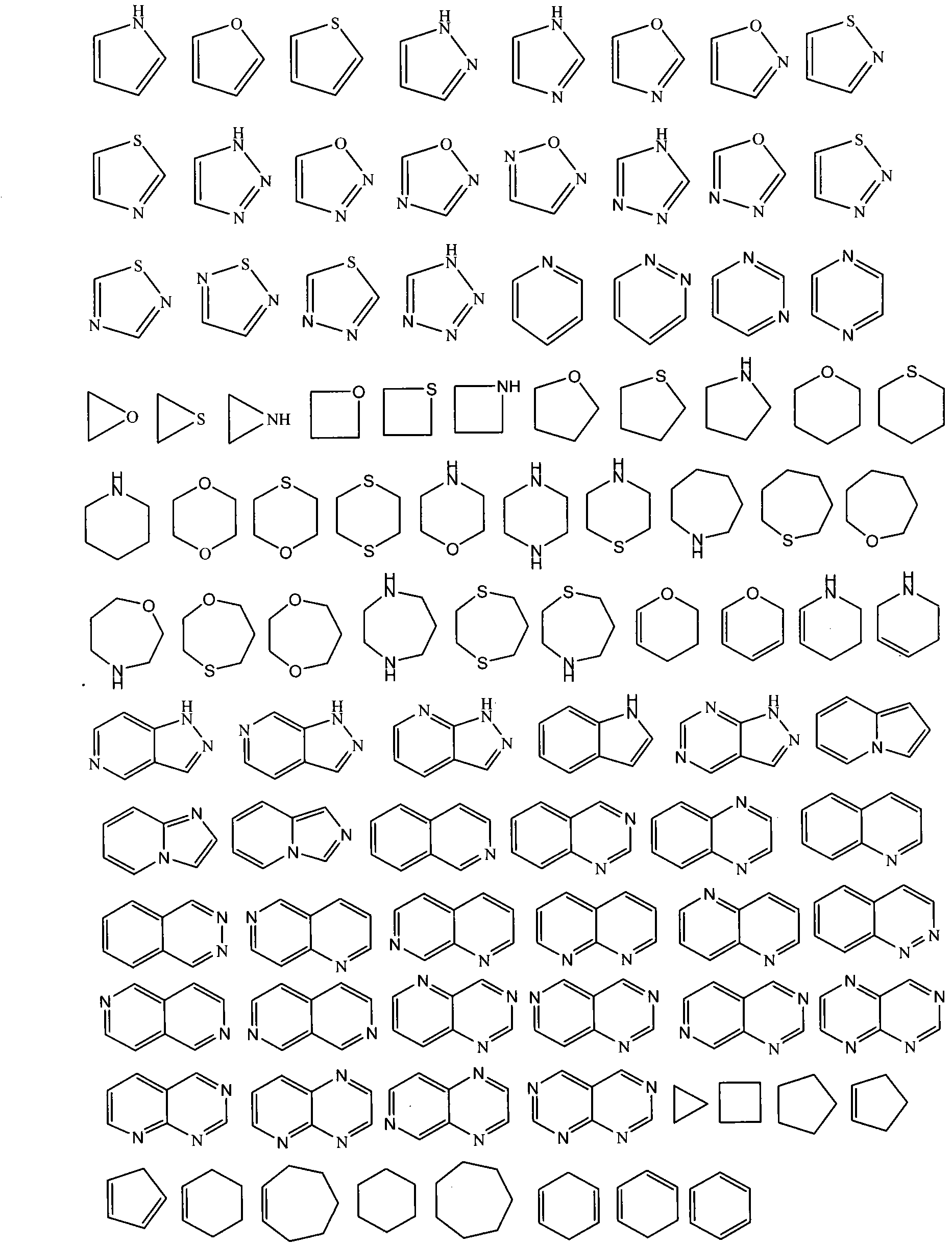
Pyrazolopyrimidinone compound and derivative as well as preparation method and application thereof
A technology of compounds and solvates, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: the preparation of the compound shown in formula 3a-1
[0095] Add ethanol (1000mL), compound shown in formula 2a (27.8 g, 0.2 mol) and compound shown in formula 1a-1 (21.1 g, 0.1 mol) in sequence in the three-necked flask, stir and reflux the resulting solution for 12 hours, pass through a thin layer Chromatography (TLC) point plate test reaction is complete (developing agent is CH 2 Cl 2 / MeOH=10:1 (V / V)). After the reaction was complete, the mixture was concentrated under vacuum to obtain the crude product of the compound shown in formula 3a as a brownish yellow oily liquid, which was dissolved with ethanol (100mL), then stirred with ethyl acetate (300mL) for 2 hours, and the temperature was controlled at 10 Recrystallized at ~15°C, filtered, and dried the solid to obtain the compound represented by formula 3a-1 (12.4 g, yield 42.4%).
Embodiment 2
[0096] Embodiment 2: the preparation of the compound shown in formula 3a-1
[0097] Add ethanol (1000mL), the compound shown in formula 2a (16.7 g, 0.12 mol) and the compound shown in formula 1a-1 (21.1 g, 0.1 mol) in sequence in the three-necked flask, and the resulting solution was stirred and refluxed for 8 hours. Chromatography (TLC) point plate test reaction is complete (developing agent is CH 2 Cl 2 / MeOH=10:1 (V / V)). After the reaction was complete, the mixture was concentrated under vacuum to obtain the crude product of the compound shown in formula 3a as a brownish-yellow oily liquid, which was dissolved with ethanol (100mL), then stirred with ethyl acetate (300mL) for 2 hours, and the temperature was controlled at 20 Recrystallized at ~25°C, filtered, and dried the solid to obtain the compound represented by formula 3a-1 (7.83 g, yield 26.8%).
Embodiment 3
[0098] Embodiment 3: the preparation of the compound shown in formula 3a-1
[0099] Add ethanol (1000mL), compound shown in formula 2a (34.8 g, 0.25 mol) and compound shown in formula 1a-1 (21.1 g, 0.1 mol) in sequence in the three-necked flask, stir and reflux the resulting solution for 24 hours, pass through a thin layer Chromatography (TLC) point plate test reaction is complete (developing agent is CH 2 Cl 2 / MeOH=10:1 (V / V)). After completion of the reaction, the mixture was concentrated under vacuum to obtain the crude product of the compound shown in formula 3a as a brownish-yellow oily liquid, which was dissolved with ethanol (100mL), then stirred with ethyl acetate (300mL) for 2 hours, and the temperature was controlled at 15 Recrystallized at ~20°C, filtered, and dried the solid to obtain the compound represented by formula 3a-1 (9.94 g, yield 34.0%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com