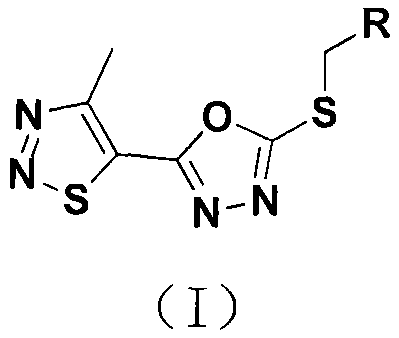
Thiadiazole-containing oxadiazole compound as well as preparation method and application thereof
A technology containing oxadiazoles and thiadiazoles, which is applied in the field of oxadiazoles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 2-(2,4-dichlorobenzylthio)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazole 1
[0024] Yield 86%, melting point 85-86°C; 1 H NMR (CDCl 3 , 400MHz), δ: 3.06(s, 3H, Het-Me), 4.60(s, 2H, SCH 2 ), 7.22 (d, J=8.49Hz, 1H, ArH), 7.44 (s, 1H, ArH), 7.61 (d, J=8.27Hz, 1H, ArH). MS (ESI), m / z: 360 ( M+1).
Embodiment 2
[0026] 2-(3-Chlorobenzylthio)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazole 2
[0027] Yield d73.5%, melting point 88-89°C; 1 H NMR (CDCl 3 , 400MHz), δ: 3.06(s, 3H, Het-Me), 4.51(s, 2H, SCH 2 ), 7.30(s, 2H, ArH), 7.34-7.39(m, 1H, ArH), 7.47(s, 1H, ArH). MS(ESI), m / z: 326(M+1).
Embodiment 3
[0029] 2-(3-fluorobenzylthio)-5-(4-methyl-1,2,3-thiadiazol-5-yl)-1,3,4-oxadiazole 3
[0030] Yield 88%, melting point 63-64°C; 1 H NMR (CDCl 3 , 400MHz), δ: 3.06(s, 3H, Het-Me), 4.53(s, 2H, SCH 2 ), 7.04(t, J=7.46Hz, 1H, ArH), 7.19(d, J=9.27Hz, 1H, ArH), 7.29-7.35(m, 2H, ArH). MS (ESI), m / z: 309(M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com