Method and equipment for continuously producing epsilon-caprolactam
A technology of caprolactam and cyclohexanone oxime, applied in the field of continuous production of ε-caprolactam and equipment, can solve the problem of energy consumption in the distillation area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
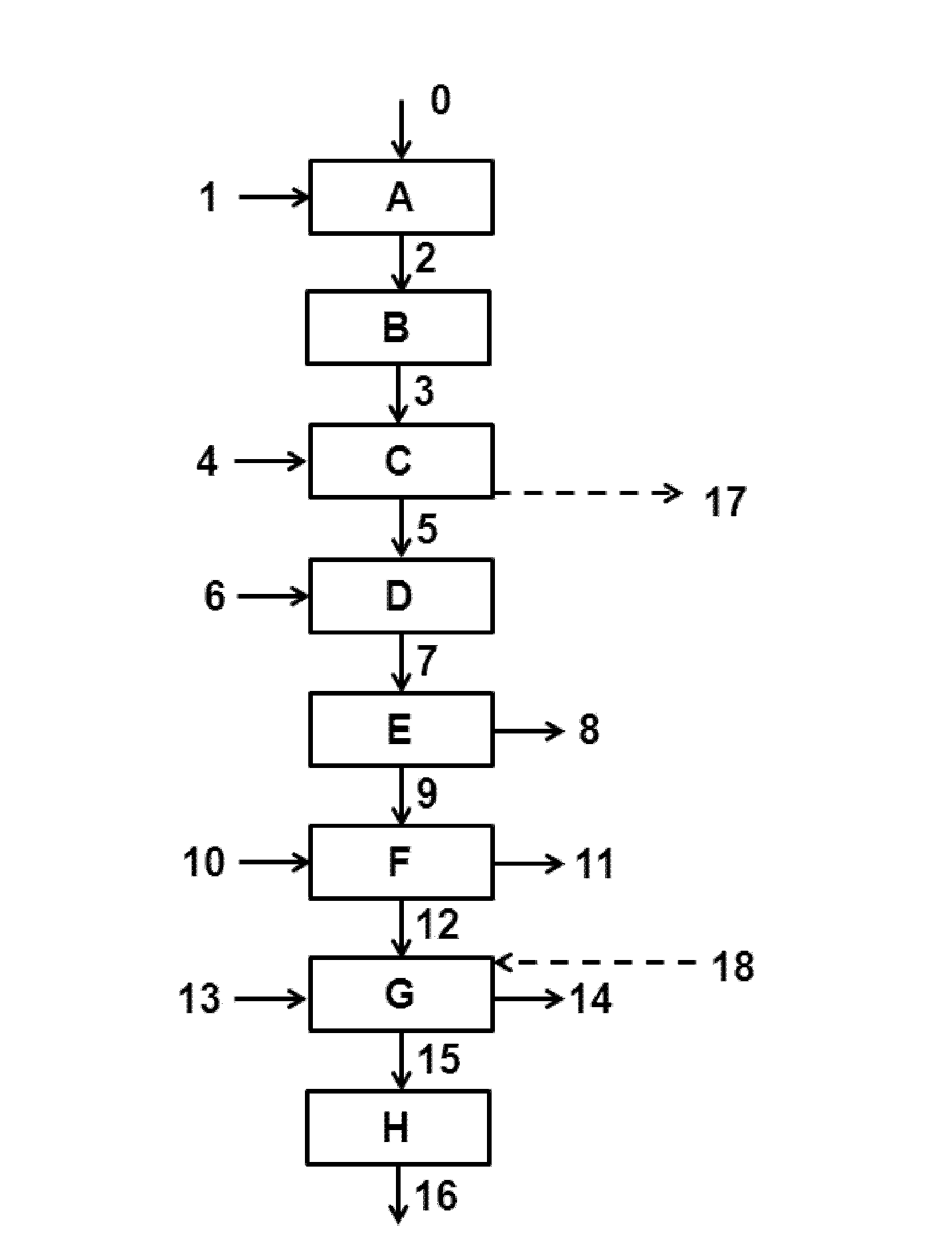
[0067] use Figure 4 the device. The heat generated in the first stage of the Beckmann rearrangement reaction zone was used as an energy source for the separation of a mixture containing ε-caprolactam and benzene in a two-step distillation process.
[0068] Cyclohexanone oxime was added to the tertiary Beckmann rearrangement reaction zone. 19 ton / hr cyclohexanone oxime was fed to the first stage mixture plant [K] via line [102]. Input 25 ton / hr oleum (sulfuric acid and SO 3 ). The heat removed from the ε-caprolactam-containing mixture in the in-process heat exchanger [N] was about 13 MW. The temperature of the ε-caprolactam-containing mixture entering the in-process heat exchanger [N] was 96°C and the temperature of the ε-caprolactam-containing mixture leaving the in-process heat exchanger [N] was 75°C.
[0069] The third-stage ε-caprolactam-containing mixture leaving the Beckmann rearrangement reaction zone was neutralized with aqueous ammonia, thereby obtaining a two-ph...
Embodiment 2
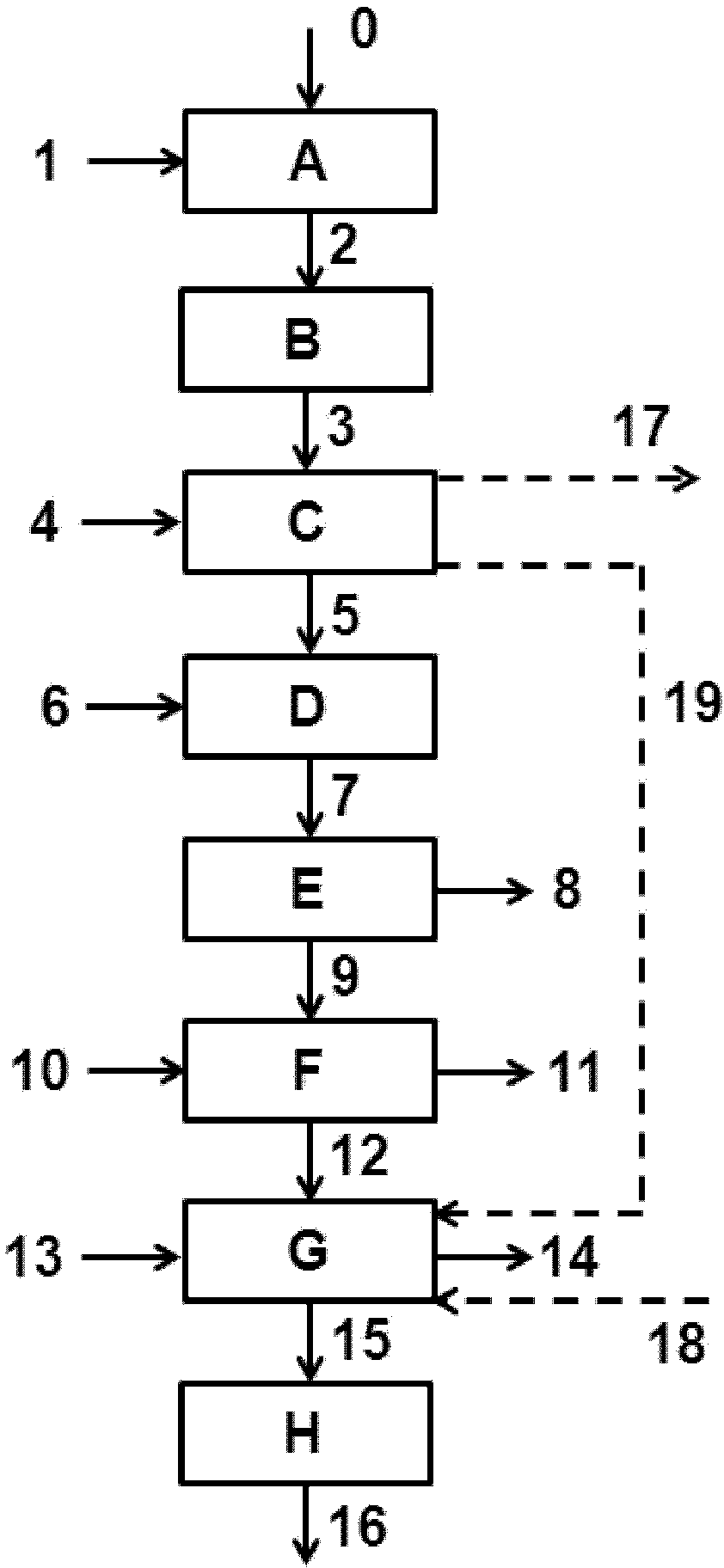
[0080] use image 3 Said plant, where the only difference is that the feed to the distillation column, a 148 ton / hr benzene mixture containing 18.3 wt.% ε-caprolactam, is not fed into line [208] but is fed from the distillation column [P] The top of the column is added to the theoretical plate 4 ( image 3 not shown). The heat generated in the first stage of the Beckmann rearrangement reaction zone was used as an energy source for the separation of a mixture containing ε-caprolactam and benzene in a one-step distillation process.
[0081] Cyclohexanone oxime was added to the tertiary Beckmann rearrangement reaction zone. Input 19 ton / hr cyclohexanone oxime via line [102] phase first stage mixing device [K]. Input 25 ton / hr oleum (sulfuric acid and SO 3 ). The heat removed from the ε-caprolactam-containing mixture in the in-process heat exchanger [N] was about 13 MW. The temperature of the ε-caprolactam-containing mixture entering the in-process heat exchanger [N] was 96°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com