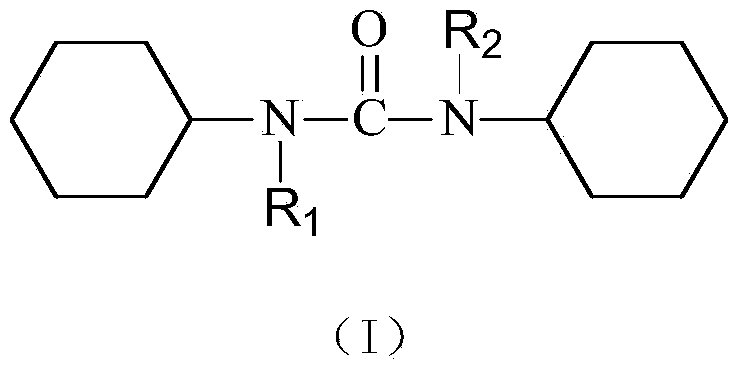
N,N'-dicyclohexyl-N-higher fatty acid ureide analogs and pharmaceutical application thereof
A fatty acid urea, dicyclohexyl technology, applied in the N field, can solve the problems of in vitro experiment influence, poor water solubility, etc., and achieve the effects of reducing dosage and toxic side effects, reducing infiltration, and reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
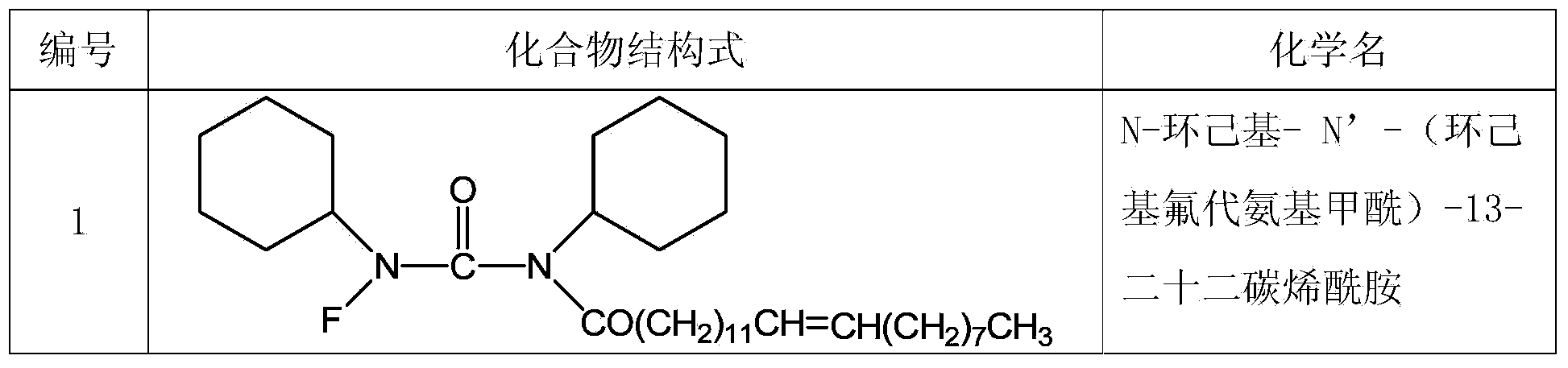
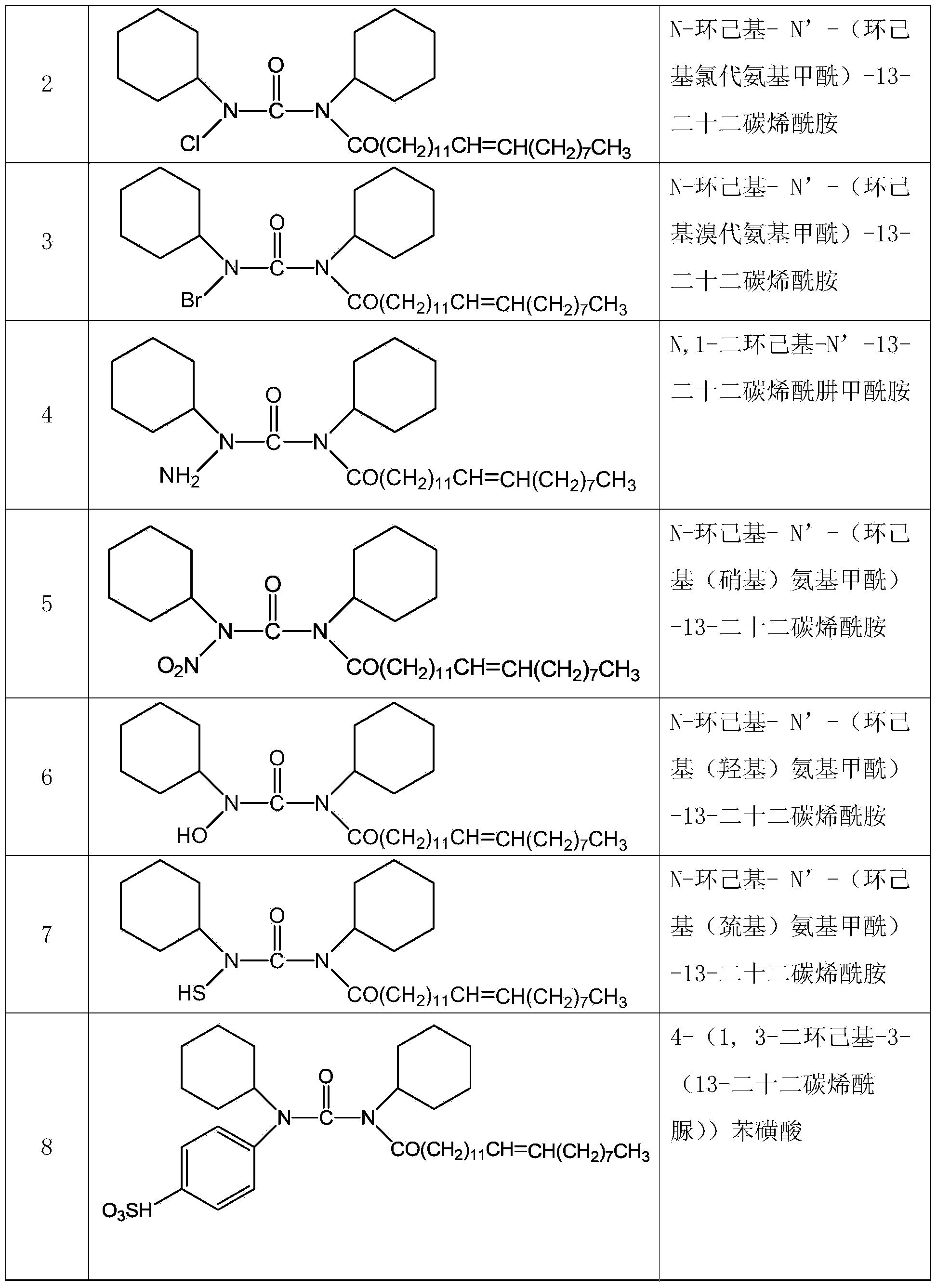
[0034] Example 1 The numbers of the compounds designed in the present invention are shown in Table 1.
[0035] Table 1 Design compound number of the present invention
[0036]
[0037]
[0038]
[0039]
[0040]
[0041]
[0042]
[0043]
Embodiment 2
[0044] Example 2 In vitro inhibition of T lymphocyte transformation experiment
[0045] This example is carried out using methods well known to those skilled in the art. The mouse spleen lymphocyte suspension was prepared by grinding method, the B cells were removed by nylon wool column, the eluted medium containing T cells was collected, centrifuged at 1800 rpm for 5 min; Suspend T cells (purity > 90%), count, adjust the cell concentration, according to 1 × 10 per well 6 Each was added to a 96-well culture plate, and then 5 μg / ml concanavalin A stimulator was added to stimulate the proliferation and differentiation of T cells, and a certain concentration of compounds of the present invention including numbers 1 to 48 were added, and the total volume of the culture solution in each well was 200 μl. Put in 5%CO 2 After culturing in the incubator for 72 hours, the proliferation of cells in each well was detected by BrdU assay, and the OD450 of each well was detected by a micro...
Embodiment 3
[0046] Example 3 In vitro inhibition of mixed lymphocyte culture experiment
[0047] This example is carried out using methods well known to those skilled in the art. The spleen lymphocyte suspensions of BALB / c mice and C57BL / 6 mice were prepared by grinding method, the B cells were removed by nylon wool column, the eluted culture medium containing T cells was collected, centrifuged at 1800 rpm for 5 min; Resuspend T cells (purity > 90%) in RPMI-1640 medium of bovine serum, count, adjust cell concentration, and obtain T lymphocytes. BALB / c mouse T lymphocytes were used as stimulator cells. 5 86-well culture plates were added to each well, and C57BL / 6 mouse T lymphocytes were used as reaction cells. 6 Each well was added to a 96-well culture plate, and at the same time, a certain concentration of the compounds of the present invention including numbers 1 to 48 was added, and the total volume of the culture solution in each well was 200 μl. Put in 5%CO 2 After culturing in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com