Method for preparing tylosin macrolide and derivatives thereof
A technology of tylosin and macrolide, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of product purity and yield decline, lactone bond hydrolysis, etc., and reduce the synthesis cost, increase yield, and the effect of process operation stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
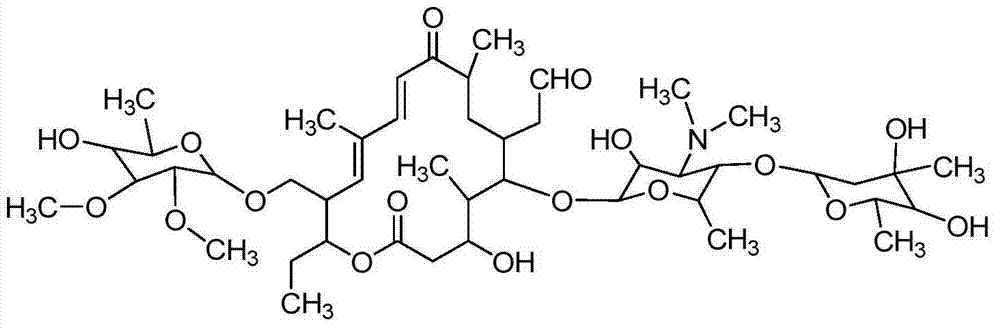
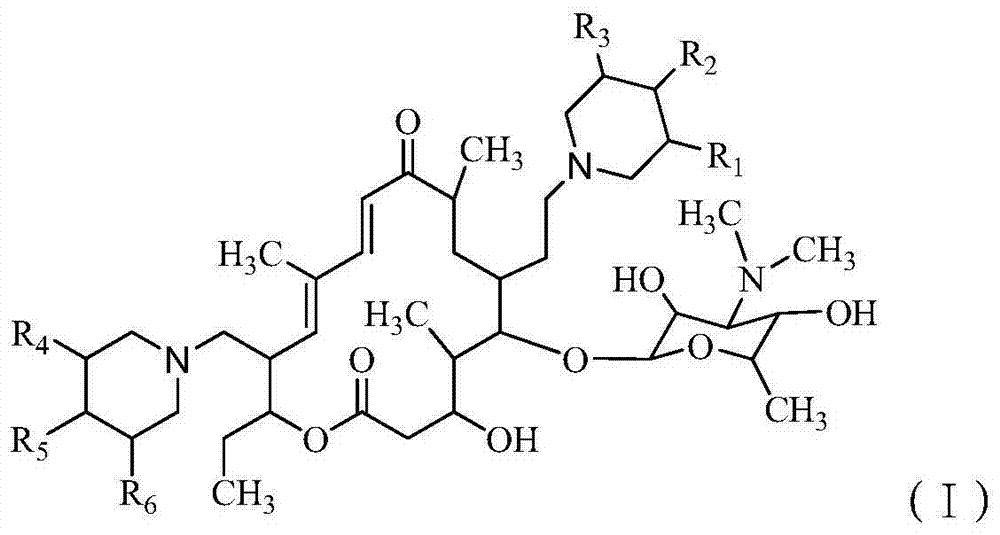
[0150] Preparation of 20,23-dipiperidinyl-5-O-mycaminosyl-tylonolide from tylosin A.
[0151]
[0152] Part A. Reductive amination and acid hydrolysis of mycaminosoxy substituents. 23-O-(6-deoxy-2,3-di-O-methyl-D-allosyl)-20-piperidinyl-5-O-mycaminosyl-tylonolide compound ( 2) Preparation.
[0153]
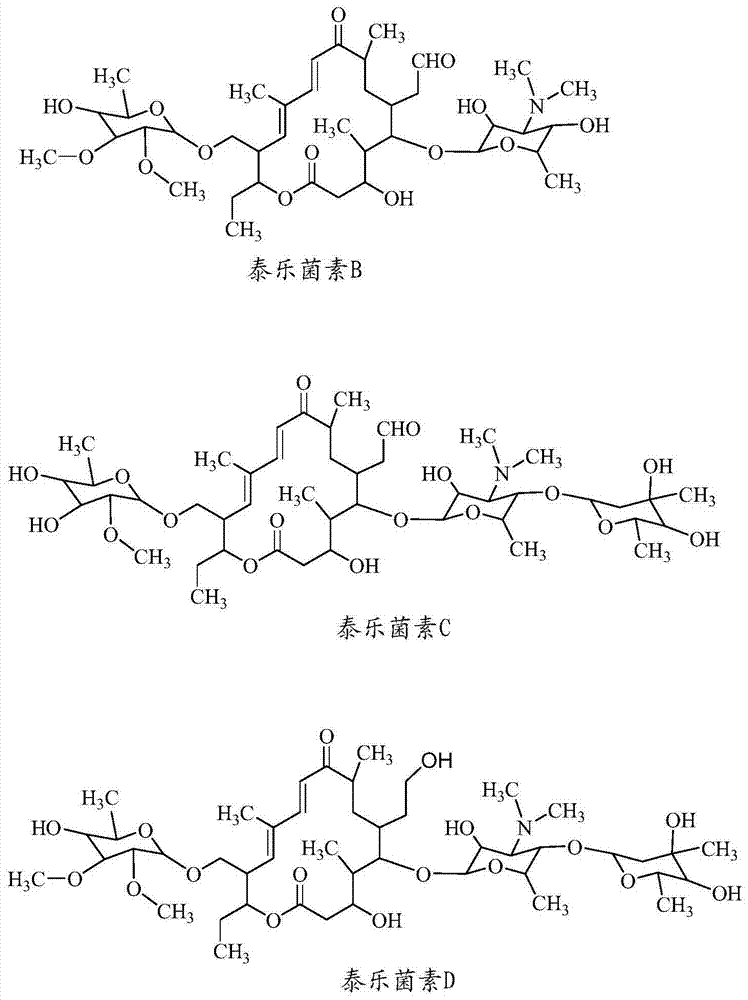
[0154] Toluene (1431g), tylosin A (1) (229g; tylosin A ≥ 80%, and tylosin A, B, C and D ≥ 95%), piperidine (25.5g) and Formic acid (67.5 g) was charged to the reactor. The mixture was heated to 65-75°C with stirring and kept at this temperature for 4-5 hours. Take a small sample and evaporate the solvent to dryness, and monitor 23-O-(6-deoxy-2,3-di-O-methyl-D-allosyl)-20-piperidinyl-5-O-carbamycin by HPLC Formation of the glycosyl-tylonolide compound (2). After the reaction was complete (tylosin A (1) HPLC content ≤ 2%), the product mixture was cooled to ambient temperature.
[0155] Part B. Acid hydrolysis of 6-deoxy-2,3-di-O-methyl-D-allosyl substituent and 23-hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com