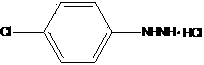
Novel synthesis process of P-chlorophenylhydrazine hydrochloride
A technology for the synthesis of chlorophenylhydrazine hydrochloride, applied in hydrazine preparation, organic chemistry, etc., can solve the problems of asphalt-like by-products, low temperature for reaction, cumbersome operation, etc., achieve low operating cost, convenient temperature control, Effects with simple processing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
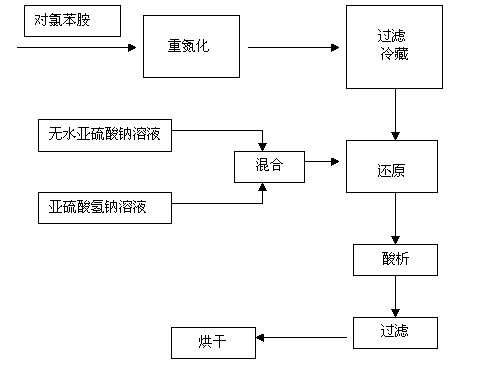
[0027] Step 1: Add 0.555 mol of hydrochloric acid, 60 mL of water, and 0.225 mol of p-chloroaniline to the reaction flask, heat up to 60-70°C, and cool down slowly to 0-5°C after all p-chloroaniline hydrochloride is dissolved. ℃, slowly drop in a solution made of 0.231mol sodium nitrite and 40mL water, finish adding dropwise for 20 minutes, stir for 30 minutes, check the reaction end point with starch potassium iodide test paper, add 0.021mol urea, stir for 10 minutes, filter and refrigerate.
[0028] The second step: in the reaction bottle, under strong stirring, dissolve 0.45mol anhydrous sodium sulfite in 120ml water, and dissolve 0.225mol sodium bisulfite in 50ml water at the same time. After the anhydrous sodium sulfite is completely dissolved, mix the two solutions to make Its pH is maintained at 6.5, and the above-mentioned diazonium salt is slowly added below the liquid level at room temperature, reacted at 80°C for 2 hours, p-chloroaniline and sodium bisulfite are 1:1,...
Embodiment 2
[0031] The reaction steps are exactly the same as in Example 1, except that for the second step, the dropping temperature is 40°C, and p-chloroaniline diazonium salt is slowly added below the liquid level for reaction, wherein the reduction temperature becomes 60°C, and the reducing agent is anhydrous The molar ratio of sodium sulfite to sodium bisulfite was 1.8:1 and the pH was maintained at 7. In the third step, the molar ratio of hydrochloric acid to p-chloroaniline is changed to 1:2.3 to keep the pH at 3. The solid yield of reddish p-chlorophenylhydrazine hydrochloride is 89.77%. The confirmation method of the compound of this article embodiment is the same as that of Example 1.
Embodiment 3
[0033] The reaction steps are exactly the same as in Example 1, except that the reduction temperature in the second step is changed to 70°C, the molar ratio of reducing agent anhydrous sodium sulfite to sodium bisulfite is 1.5:1, and the pH is maintained at 6. In the third step, the molar ratio of hydrochloric acid to p-chloroaniline is changed to 1:2.3 to keep the pH at 3. The solid yield of reddish p-chlorophenylhydrazine hydrochloride is 92.79%. The confirmation method of the compound of this article embodiment is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com