Molecular markers and their expression analysis methods for early detection of patients with pleural mesothelioma
一种患者、皮瘤的技术,应用在间皮瘤的检查领域,能够解决骨膜蛋白间皮瘤关系未知等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Preparation of anti-periostin antibody
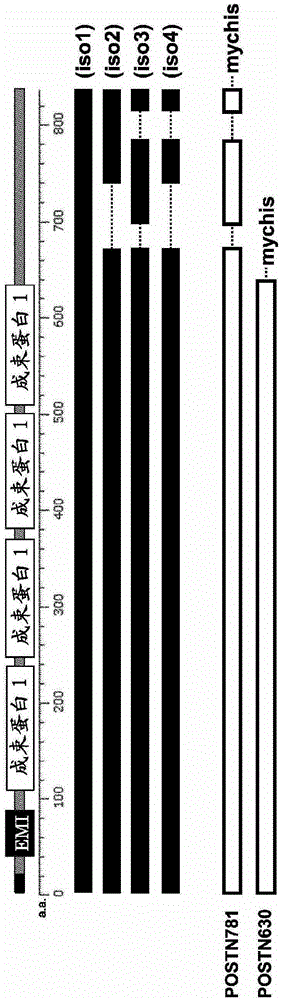
[0073] (1) Structure of human periostin protein
[0074] figure 1 It is a schematic diagram showing the position of the functional domain of the human periostin protein, the structure of each isoform, and the relationship between the structure of the immunogen used for producing the periostin antibody of the present invention. exist figure 1 In, EMI and fascin 1 denote EMILIN homology domain and fascin homology domain, respectively. iso1-4 represent isoforms 1-4 of human periostin, respectively. POSTN781 represents a recombinant protein in which a myc tag and a his tag (denoted "mychis") are fused to the carboxyl terminus of a 781 amino acid polypeptide of human periostin protein isoform 3. POSTN630 represents a recombinant protein in which a myc tag and a his tag are fused to the carboxyl terminus of a polypeptide of 630 amino acids common to all isoforms of human periostin protein comprising up to 4 fascin domains. The ami...
Embodiment 2
[0093] Determination of clinical samples
[0094] (1) Reactivity to clinical samples
[0095] The samples of healthy people were approved by the Ethics Committee of the Institute of Medical Biology of Co., Ltd.; MBL Ethics Review Committee (case number: 022, date of approval: March 27, 2007). Written measurement consent was obtained in advance from each healthy person who provided the sample. The samples of mesothelioma patients were commissioned by BMR (BioMedicalResources (unified as SeraCareLifeSciencesMilford, MA.) at the time of application) (part code: DS-763 description: MesotheliomaLot#BM203975-BM203986). The above-mentioned plasma sample diluted to 1000 times was used instead of human periostin in the sandwich ELISA method described in Example 1(7) to purify the recombinant protein. Table 2 shows the measurement results of the plasma samples of 16 healthy persons and the plasma samples of 11 mesothelioma patients for the antibody combinations shown in Table 1.
[0...
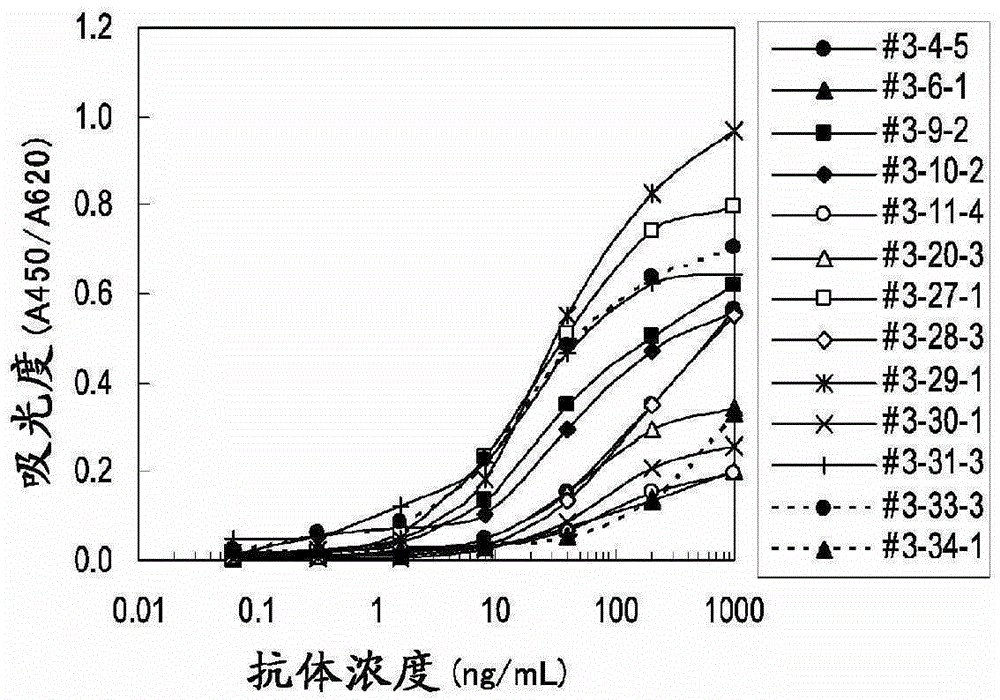
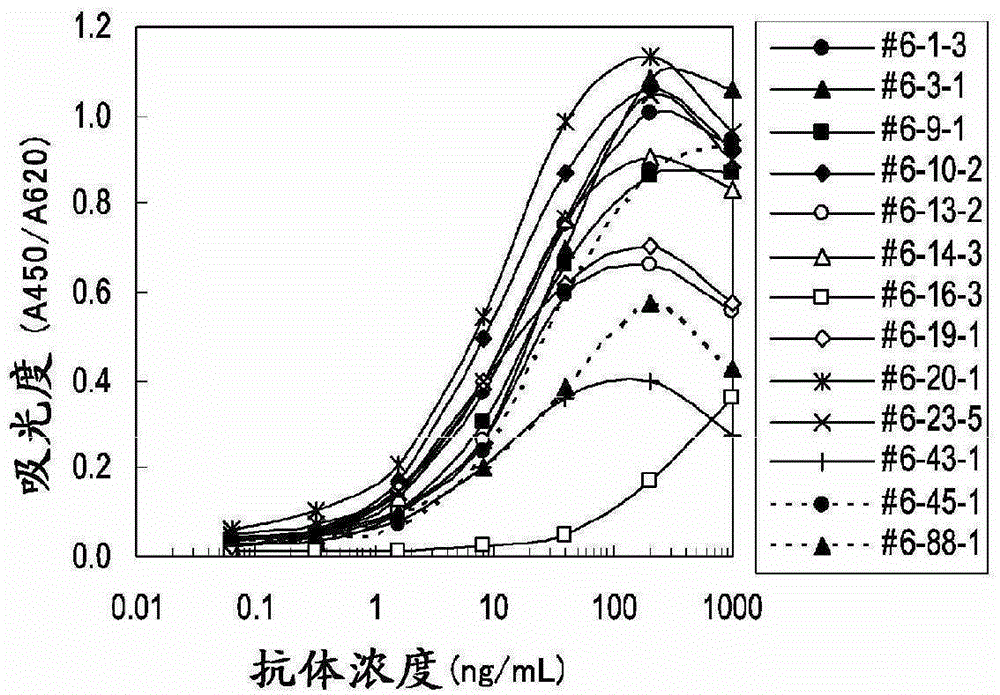
Embodiment 3
[0102] Determination of clinical samples
[0103] (1) Determination of periostin concentration in plasma samples of mesothelioma patients and healthy subjects
[0104] will be used containing 0.1M NaHCO 3 , 0.1MNa 2 CO 3Anti-human periostin monoclonal antibody #6-14-3 diluted to 10 μg / mL with a solution of 0.15% Proclin 150 (SUPELCO), and 50 μL each was coated on a MaxiSorp 96-well plate (NUNC, Thermophysics Corporation, Ltd.), Immobilize the antibody by standing at 4°C overnight or at room temperature for 2 hours. After the antibody solution was removed, 150 μL each of blocking buffer (PBS supplemented with 1% BSA (Proliant, Beritas, Inc.), 0.15% Proclin 150, and 5% sucrose) was dispensed and allowed to stand overnight at 4°C or at room temperature for 2 minutes. Hour. After removing the blocking buffer solution, PBS added with 1% BSA, 0.1% Tween20, 0.15% Proclin150 and 50 μg / mL MAK-33 was used as a diluent to prepare a periostin standard substance (POSTN781 starting fro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com