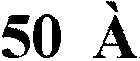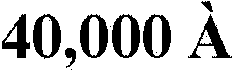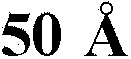Polymeric sorbent for removal of impurities from whole blood and blood products
A technology of blood products and sorbents, applied in blood diseases, medical raw materials derived from mammals, applications, etc., can solve problems such as blood shortages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: overall synthetic method
[0041] In the search for neutrally buoyant beads, early experiments showed porous and solid beads of divinylbenzene-based polymers floating on packed red blood cells. Red blood cells (alone) have a density of 1.125 g / mL, while some of our divinylbenzene porous polymers have a backbone density of 1.082 g / mL. In practice, bags of packed red blood cells contain not only cells, but also substances such as preservatives (eg SAG), and residual plasma, which can cause the density of packed red blood cells to vary from bag to bag. The density of plasma is 1.025 g / mL. In our study, we found hematocrit values of 40% to 60%. Interestingly, poly-4-chlorostyrene has a density of 1.55 g / mL. Therefore, consider that the copolymerization of divinylbenzene and 4-chlorostyrene at specific ratios will generate a series of neutrally buoyant beads in packed red blood cells. Such asfigure 1 It was shown that increasing the amount of 4-chlorostyr...
Embodiment 2~4
[0043] Embodiment 2~4: Sorbent synthesis
[0044] Three porous polymeric sorbents were characterized by their pore structure and their synthesis (described in Examples 2, 3 and 4). Structural characterization is given in Examples 5, 6 and 7. The polymers synthesized in these steps are then placed into suitable blood containers.
[0045] The synthesis procedure consists of (1) preparation of the aqueous phase, (2) preparation of the organic phase, (3) implementation of the suspension polymerization, (4) purification of the resulting porous polymer adsorbent product (work-up), and (5) adding a hemocompatible coating.
[0046] Reactor setup. 0.5 L kettle reactor equipped with an over-head stirrer with multi-level stirrer blade, water-cooled condenser, thermocouple, and bubbler (bubbler). A gasket was installed between the top cover and bottom kettle. All unused ports were capped with suitable stoppers. The temperature was controlled with a heating mantle regulated by a tem...
Embodiment 5
[0053] Embodiment 5: Pore structure characterization
[0054] The pore structure of the sorbent polymer was measured with Micromeritics AutoPore IV9500V1.09 Mercury Penetrometer (Mercury Penetrometer) (pressure Hg instrument) or Micromeritics ASAP2010 instrument (N 2 desorption) analysis to reveal the presence of large and small pores. The results are provided in Figure 4 and Figure 5 , where the pore volume is plotted as a function of pore diameter. Figure 7 It is shown in that different pore structures allow the polymer to sorb a range of different harmful by-products in blood.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com