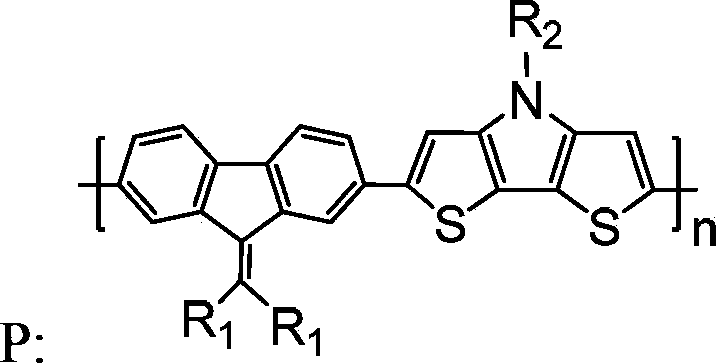
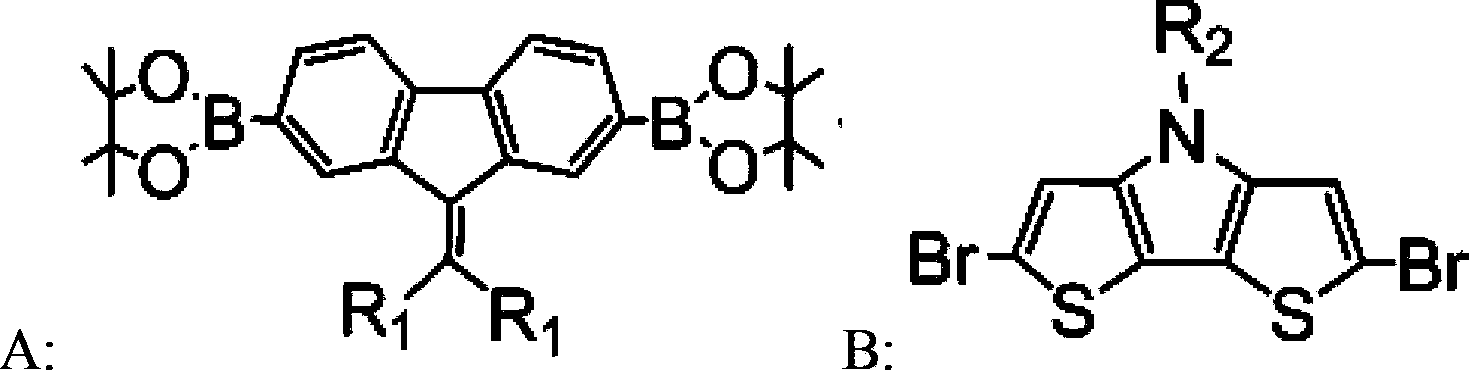
Copolymer containing alkylene fluorene and dithiophene-pyrrole, and preparation method and applications thereof
A phenopyrrole copolymer and alkylene fluorene-containing technology, which is applied in semiconductor/solid-state device manufacturing, organic chemistry, electric solid-state devices, etc., can solve device solar radiation spectrum mismatch, low energy conversion efficiency, and low current carrying capacity To improve photoelectric conversion efficiency, increase transmission speed and efficiency, and increase order and regularity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A copolymer containing alkylene fluorene and dithienopyrrole, that is, poly[(9-di(dodecyl)methylenefluoren-2,7-yl-)-(4-octyl-dithieno Pyrrol-2,6-yl-)] (n=10), denoted as copolymer P1, the structural formula is as follows:
[0044]
[0045] The preparation steps of P1 are as follows:
[0046] 1. Preparation of compound 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9-bis(dodecyl)methylene fluorene, Recorded as A1 (structural formula is as follows).
[0047]
[0048] (1) Preparation of compound 2,7-dibromo-9-bis(thiomethyl)methylenefluorene, the reaction formula is:
[0049]
[0050] At room temperature under the protection of nitrogen, add 1L of anhydrous dimethyl sulfoxide (DMSO) dissolved in 0.15mol 2,7-dibromofluorene into the reaction flask, and add 0.30mol tert-butanol under the condition of uniform stirring Potassium, then inject 0.15mol carbon disulfide with a syringe and stir to react for 10 minutes, then slowly add 0.15mol methyl iodide dropwise wi...
Embodiment 2
[0063] A copolymer containing alkylene fluorene and dithienopyrrole, that is, poly[(9-bis(2-ethylhexyl)methylenefluoren-2,7-yl-)-(4-(1-(2 -Ethylhexyl)-3-ethyl-heptyl)-dithienopyrrole-2,6-yl-)] (n=100), denoted as copolymer P2, the structural formula is as follows:
[0064]
[0065] The preparation steps of P2 are as follows:
[0066] 1. Preparation of compound 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9-bis(2-ethylhexyl)methylenefluorene , denoted as A2 (structural formula is as follows).
[0067]
[0068] (1) Preparation of compound 2,7-dibromo-9-bis(thiomethyl)methylenefluorene, the reaction formula is:
[0069]
[0070] At room temperature under the protection of nitrogen, add 1L anhydrous DMSO dissolved with 0.3mol 2,7-dibromofluorene into the reaction flask, add 0.72mol potassium tert-butoxide under the condition of uniform stirring, and then inject 0.36 mol of carbon disulfide was stirred and reacted for 20 minutes, and then 0.36mol of methyl iodide wa...
Embodiment 3
[0083] A copolymer containing alkylene fluorene and dithienopyrrole, poly[(9-di(butyl)methylenefluoren-2,7-yl-)-(4-tetradecyl-dithienothio Sorrel-2,6-yl-)] (n=73), denoted as copolymer P3, the structural formula is as follows:
[0084]
[0085] The preparation steps of P3 are as follows:
[0086] 1. Preparation of compound 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)-9-dibutylmethylene fluorene, denoted as A3 ( The structural formula is as follows).
[0087]
[0088] (1) Preparation of compound 2,7-dibromo-9-bis(thiomethyl)methylenefluorene, the reaction formula is:
[0089]
[0090] At room temperature under the protection of nitrogen, add 1L anhydrous DMSO dissolved with 0.02mol 2,7-dibromofluorene into the reaction flask, add 0.044mol potassium tert-butoxide under the condition of uniform stirring, and then inject 0.022 mol of carbon disulfide was stirred and reacted for 15 minutes, and then 0.022mol of methyl iodide was slowly added dropwise with a dropping...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com