n, n-disubstituted benzazine heterocyclo-2-amine compounds and uses thereof
A technology of amine compounds and benzonitrogen, which is applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problems of low brain penetration ability, extremely poor membrane permeability, easy degradation in vivo, and limited therapeutic use, etc., and achieve strong inhibition active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
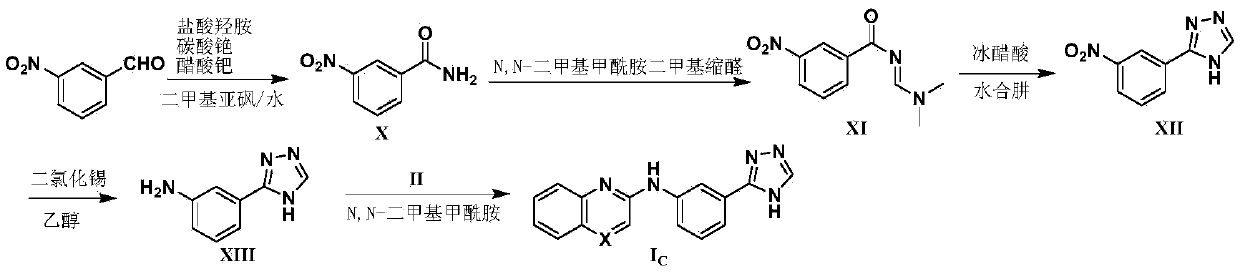
Image
Examples
Embodiment 1
[0080] Preparation of 2-Chloroquinoxaline (Intermediate II-1).
[0081]
[0082] Dissolve 2.000g of 2-hydroxyquinoxaline in 20mL of phenylphosphonyl dichloride, and react under reflux at 110°C for 12h under nitrogen protection. The reaction was monitored by TLC. After the reaction was complete, the reaction solution was cooled to room temperature, slowly poured into ice water and kept stirring. After the product was precipitated, it was suction filtered, washed with water, and dried to obtain 1.830 g of 2-chloroquinoxaline (intermediate II-1), with a yield of 81%.
[0083] 1 H-NMR (400MHz, CDCl 3 ):δ8.54(s,1H),7.92–7.81(m,1H),7.80–7.72(m,1H),7.63–7.50(m,2H).
Embodiment 2
[0085] Preparation of 2-Chloroquinoline (Intermediate II-2).
[0086]
[0087] Except for replacing 2-hydroxyquinoxaline with 2-hydroxyquinoline, the other required raw materials, reagents and preparation methods are the same as in Example 1 to obtain 1.911g of intermediate 2-chloroquinoline (intermediate II-2). The rate is 85%.
[0088] 1 H-NMR (400MHz, CDCl 3 ):δ8.14(d,J=8.6Hz,1H),8.08(d,J=8.5Hz,1H),7.83(d,J=8.2Hz,1H),7.80–7.73(m,1H),7.58 (m,1H),7.41(d,J=8.6Hz,1H).
Embodiment 3
[0090] Preparation of ethyl m-nitrocinnamate (Intermediate III).
[0091]
[0092] Dissolve 2.000g of m-nitrocinnamic acid in 40mL of ethanol, slowly add 1mL of concentrated sulfuric acid dropwise, and reflux for 12h under nitrogen protection. The reaction was monitored by TLC. After the reaction, the reaction system was cooled to room temperature, the solvent was evaporated under reduced pressure, the reaction system was adjusted to alkaline with saturated sodium bicarbonate solution, extracted with ethyl acetate, the organic layer was taken, dried, suction filtered, and evaporated under reduced pressure. solvent to obtain a crude product. After separation and purification by column chromatography, 2.000 g of intermediate ethyl m-nitrocinnamate (intermediate III) was obtained, with a yield of 88%.
[0093] 1 H-NMR (400MHz, CDCl 3 ):δ7.62(d,J=15.8Hz,1H),7.20(t,J=7.6Hz,1H),6.98(d,J=7.8Hz,1H),6.88(s,1H),6.76(d ,J=8.1Hz,1H),6.40(d,J=16.2Hz,1H),4.28(q,J=7.2Hz,2H),1.36(t,J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com